554030
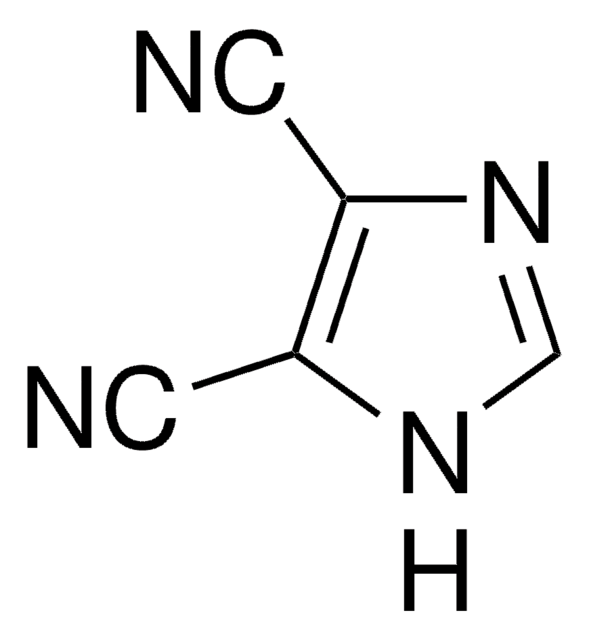
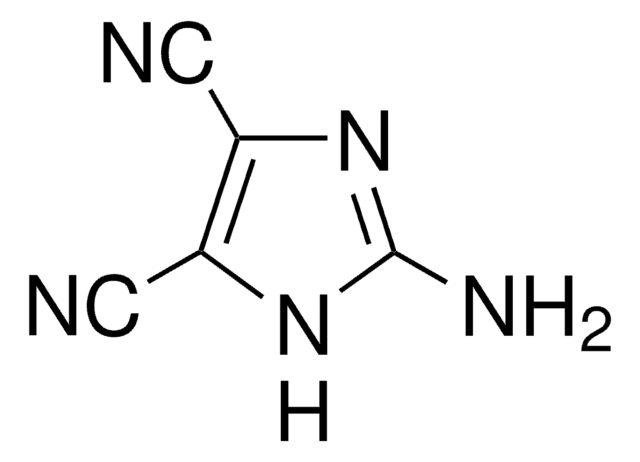
4,5-Dicyanoimidazole
99.0%
Synonym(s):
4,5-Imidazoledicarbonitrile
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H2N4
CAS Number:
Molecular Weight:
118.10
Beilstein:
118208
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99.0%
mp
168-175 °C (lit.)
functional group
nitrile
SMILES string
N#Cc1nc[nH]c1C#N
InChI
1S/C5H2N4/c6-1-4-5(2-7)9-3-8-4/h3H,(H,8,9)
InChI key
XGDRLCRGKUCBQL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4,5-Dicyanoimidazole (DCI) participates as an activator in the synthesis of oligonucleotides.
Application
4,5-Dicyanoimidazole may be used to synthesize:
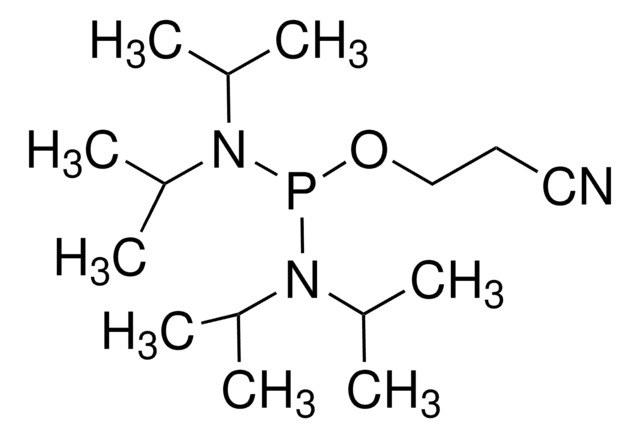
- 5′-O-(4,4′-dimethoxytrityl)-2′-deoxythymidine 3′-O-(2-cyanoethyl N,N-diisopropylphosphoramidite)
- 4,5-di(amidoximyl)imidazole [4,5-(DAO)Im]
- 1-(4-methoxybenzyl)-4,5-dicyanoimidazole
- novel imidazo[4 5-e][1 3]diazepine analog
- N-benzyl-4,5-dicyanoimidazole]
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 1-benzyl-8, 9-dihydroimidazo [4, 5-c] pyrrolo [3, 2-g] quinolin-4 (5H)-one via palladium-catalyzed intramolecular arylation.
Delest B, et al.
Tetrahedron, 60(29), 6079-6083 (2004)
4, 5-Dicyanoimidazole.
Sebesta DP and Vagle K.
e-EROS Encyclopedia of Reagents for Organic Synthesis. null
C Vargeese et al.
Nucleic acids research, 26(4), 1046-1050 (1998-03-21)
A new activator for the coupling of phosphoramidites to the 5'-hydroxyl group during oligonucleotide synthesis is introduced. The observed time to complete coupling is twice as fast with 4, 5-dicyanoimidazole (DCI) as the activator, compared with 1 H -tetrazole. The
Structural clues to UO₂²⁺/VO₂⁺ competition in seawater extraction using amidoxime-based extractants.
Steven P Kelley et al.
Chemical communications (Cambridge, England), 50(83), 12504-12507 (2014-09-06)
Here we present the first structural comparison of amidoxime complexes of UO2(2+) and VO2(+) (the main competitor in the extraction of uranium from seawater using amidoxime-based sorbents) using a 4,5-di(amidoxime)-functionalized imidazole ligand. The amidoxime groups resist tautomerization in both cases
Matthew T Holden et al.
Analytical chemistry, 87(22), 11420-11428 (2015-10-24)
The photolithographic fabrication of high-density DNA and RNA arrays on flexible and transparent plastic substrates is reported. The substrates are thin sheets of poly(ethylene terephthalate) (PET) coated with cross-linked polymer multilayers that present hydroxyl groups suitable for conventional phosphoramidite-based nucleic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service