537365
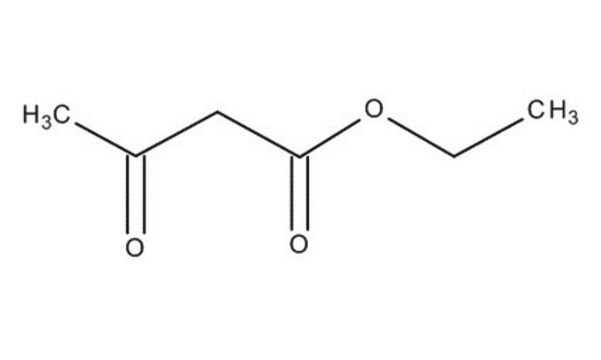
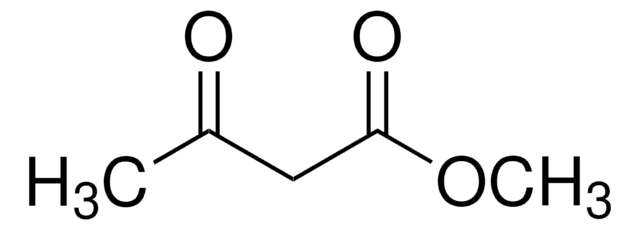
Methyl acetoacetate
ReagentPlus®, ≥98.5% (GC)
Synonym(s):
MAA, 3-Oxobutanoic acid methyl ester, Acetoacetic acid methyl ester
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
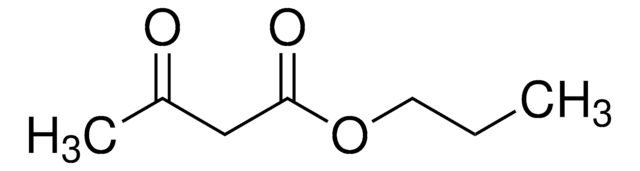
CH3COCH2COOCH3
CAS Number:
Molecular Weight:
116.12
Beilstein:
506727
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
≥98.5% (GC)
autoignition temp.
536 °F
manufacturer/tradename
Sigma-Aldrich
refractive index
n20/D 1.419 (lit.)
bp
169-170 °C/70 mmHg (lit.)
mp
−80 °C (lit.)
density
1.076 g/mL at 25 °C (lit.)
functional group
ester
ketone
SMILES string
COC(=O)CC(C)=O
InChI
1S/C5H8O3/c1-4(6)3-5(7)8-2/h3H2,1-2H3
InChI key
WRQNANDWMGAFTP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Methyl acetoacetate (MAA) can be used as a reactant for:
- Transesterification with alcohols by using various catalysts.
- The synthesis of 4-methylcoumarins with phenol in presence of zinc and I2 as catalysts.
- Asymmetric heterogeneous hydrogenation reactions by transition metal catalysts.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
158.0 °F - closed cup
Flash Point(C)
70 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synergism between microwave and enzyme catalysis in intensification of reactions and selectivities: transesterification of methyl acetoacetate with alcohols.
Yadav GD and Lathi PS
J. Mol. Catal. A: Chem., 223(1-2), 51-56 (2004)
Synthesis and studies of 6, 6′ -BINAP derivatives for the heterogeneous asymmetric hydrogenation of methyl acetoacetate.
Saluzzo C, et al.
Tetrahedron Asymmetry, 13(11), 1141-1146 (2002)
Enantioselective hydrogenation of methyl acetoacetate catalyzed by nickel supported on activated carbon or graphite.
Wolfson A, et al.
Applied Catalysis A: General, 208(1-2), 91-98 (2001)
Zinc mediated transesterification of ?-ketoesters and coumarin synthesis.
Chavan SP, et al.
Tetrahedron Letters, 43(47), 8583-8586 (2002)
S Aramaki et al.
Journal of inherited metabolic disease, 14(1), 63-74 (1991-01-01)
The concentrations of 2-methylacetoacetate, 2-methyl-3-hydroxybutyrate and tiglylglycine were determined by gas chromatography-mass spectrometry in urine collected before and for 8 h after loading with 100 mg of isoleucine per kg of body weight. The sum of 2-methylacetoacetate and 2-butanone, a
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 537365-1KG | 4061832565637 |
| 537365-100G | 4061832565620 |
| 537365-5G |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service