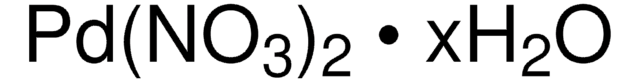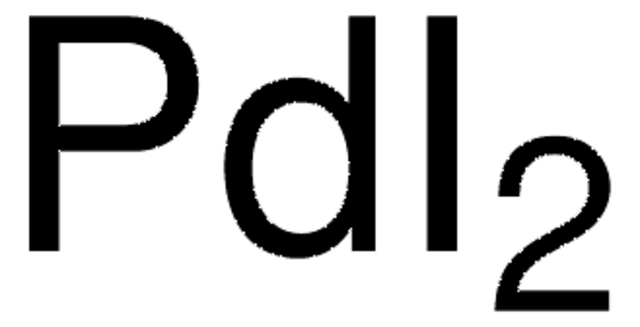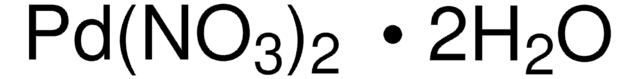520659
Palladium(II) chloride
≥99.9%
Synonym(s):
Dichloropalladium, Palladium dichloride, Palladous chloride
About This Item
Recommended Products
Quality Level
Assay
≥99.9%
composition
Pd, 59-60%
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
678-680 °C (lit.)
density
4 g/mL at 25 °C (lit.)
SMILES string
Cl[Pd]Cl
InChI
1S/2ClH.Pd/h2*1H;/q;;+2/p-2
InChI key
PIBWKRNGBLPSSY-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Palladium(II) chloride is used as an oxidizing agent and catalyst for the Suzuki-Miyaura and Mizoroki-Heck reactions.
Application
Used in the synthesis of semiconducting metal-containing polymers in which the polypyrrole backbone has a conformational energy minimum and is nearly planar.
- As catalyst for the carbonylation of organic tellurides by reaction with carbon monoxide.
- As a catalyst along with Cu(II) for the deamination of phenethylamines to phenyl substituted pyrroles.
- Together with PEG 300, promoted efficient Suzuki-coupling of aryl chlorides with aryl boronic acids.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Met. Corr. 1 - Skin Sens. 1
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 520659-100G | 4061833301487 |
| 520659-25G | 4061832546995 |
| 520659-1G | 4061832546988 |
| 520659-5G | 4061832547008 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service