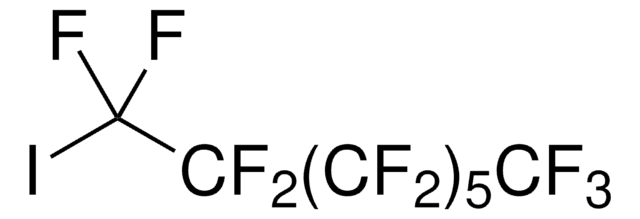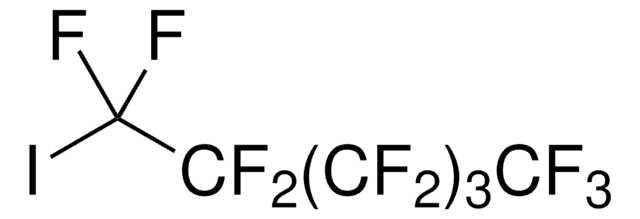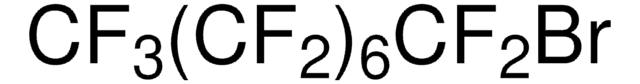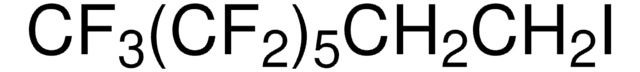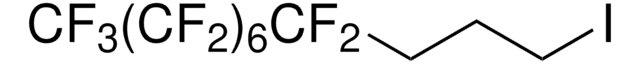446882
Perfluorohexyl bromide
98%
Synonym(s):
1-Bromotridecafluorohexane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3(CF2)5Br
CAS Number:
Molecular Weight:
398.95
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
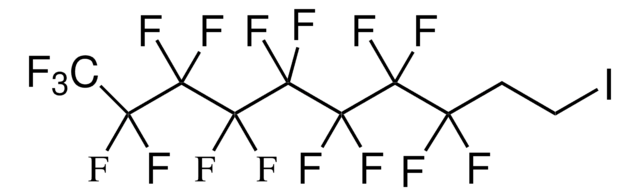
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.3 (lit.)
bp
97 °C (lit.)
density
1.871 g/mL at 25 °C (lit.)
functional group
bromo
fluoro
SMILES string
FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)Br
InChI
1S/C6BrF13/c7-5(16,17)3(12,13)1(8,9)2(10,11)4(14,15)6(18,19)20
InChI key
JTYRBFORUCBNHJ-UHFFFAOYSA-N
Application
Employed in the preparation of perfluorononanal by hydroformylation.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Intra-abdominal sepsis and perfluorocarbons: mechanism of protection.
D B Hoyt et al.
Current surgery, 39(3), 165-167 (1982-05-01)
Douglass et al.
The Journal of organic chemistry, 65(5), 1434-1441 (2000-05-18)
A triphenylsilyl group is used as an auxiliary in the synthesis of heterodisubstituted p-carboranes via triphenylsilyl-p-carborane (1). The preparation of 1 is statistical, but with recovery of the starting p-carborane, the effective conversion to 1 is about 90%. Carborane 1
S L Wootton et al.
AJR. American journal of roentgenology, 161(2), 409-416 (1993-08-01)
The disadvantages of water-soluble gastrointestinal contrast agents include high osmolality, contrast dilution, and severe toxicity if aspirated. Perfluorocarbons are nontoxic in the lung and peritoneal cavity. Because perfluorocarbons are immiscible with water, they have no osmotic effect and cannot be
Zhang Feng et al.
Angewandte Chemie (International ed. in English), 54(4), 1270-1274 (2014-12-04)
An efficient palladium-catalyzed Heck-type reaction of fluoroalkyl halides, including perfluoroalkyl bromides, trifluoromethyl iodides, and difluoroalkyl bromides, has been developed. The reaction proceeds under mild reaction conditions with high efficiency and broad substrate scope, and provides a general and straightforward access
M Scrime et al.
Journal of pharmaceutical sciences, 70(11), 1199-1201 (1981-11-01)
Beagle dogs received single perfluorohexyl bromide doses, either 30.2 g/kg po or 3.8 g/kg intratracheally. The apparent first-order plasma half-life during the terminal elimination phase was approximately 8 hr after oral treatment and greater than 8 hr after intratracheal administration.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service