390755
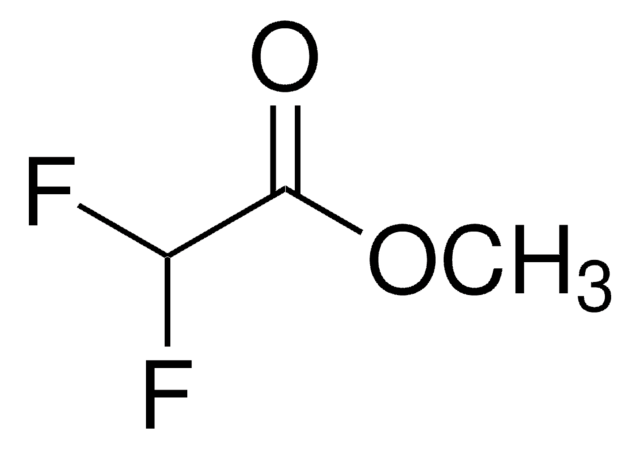
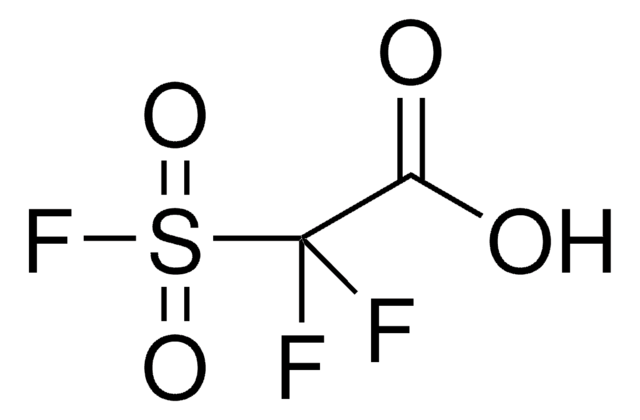
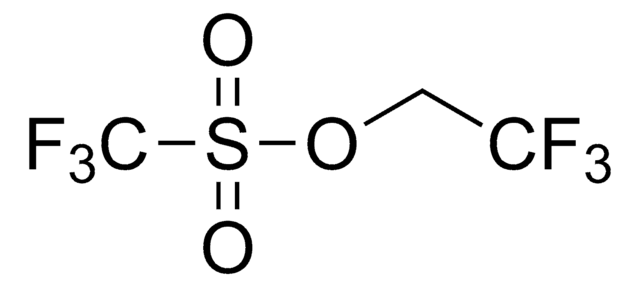
Methyl 2,2-difluoro-2-(fluorosulfonyl)acetate
97%
Synonym(s):
Difluoro(fluorosulfonyl)acetic acid methyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FSO2CF2CO2CH3
CAS Number:
Molecular Weight:
192.11
Beilstein:
1812896
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
reaction suitability
reaction type: click chemistry
refractive index
n20/D 1.351 (lit.)
bp
117-118 °C (lit.)
density
1.509 g/mL at 25 °C (lit.)
functional group
ester
fluoro
SMILES string
COC(=O)C(F)(F)S(F)(=O)=O
InChI
1S/C3H3F3O4S/c1-10-2(7)3(4,5)11(6,8)9/h1H3
InChI key
GQJCAQADCPTHKN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Useful reagent for the trifluoromethylation of alkyl halides.
- Broadly applicable reagent for perfluoroalkylations for aryl iodides and bromides
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
116.6 °F - closed cup
Flash Point(C)
47 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Journal of the Chemical Society. Chemical Communications, 705-705 (1989)
A broadly applicable copper reagent for trifluoromethylations and perfluoroalkylations of aryl iodides and bromides.
Hiroyuki Morimoto et al.
Angewandte Chemie (International ed. in English), 50(16), 3793-3798 (2011-03-29)
David Orr et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 20(44), 14305-14316 (2014-09-13)
Vinyl cyclopropane rearrangement (VCPR) has been utilised to synthesise a difluorinated cyclopentene stereospecifically and under mild thermal conditions. Difluorocyclopropanation chemistry afforded ethyl 3-(1'(2'2'-difluoro-3'-phenyl)cyclopropyl) propenoate as all four stereoisomers (18a, 18b, 22a, 22b) (all racemic). The trans-E isomer (18a), prepared in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service