382272
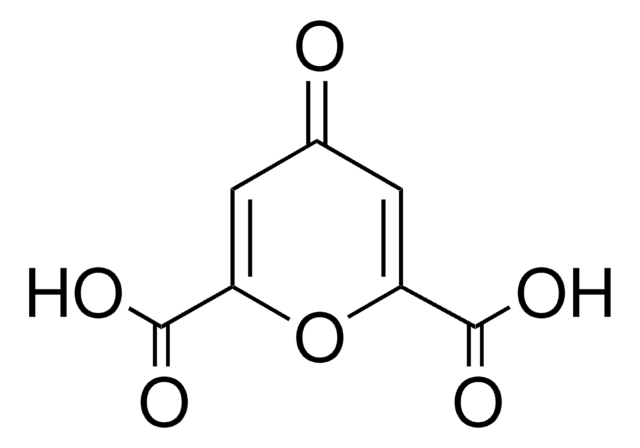
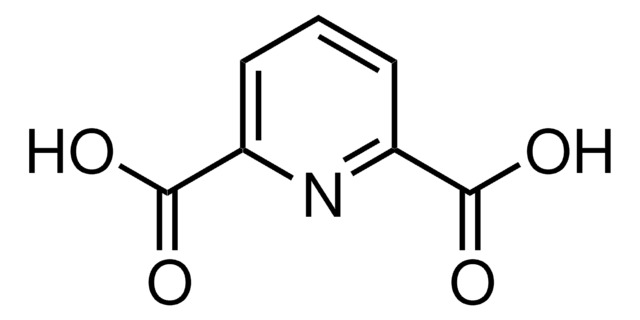
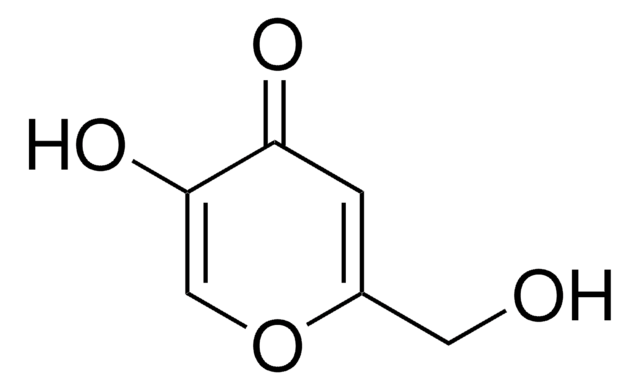
Chelidonic acid
≥95%
Synonym(s):
4-Oxo-4H-pyran-2,6-dicarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H4O6
CAS Number:
Molecular Weight:
184.10
Beilstein:
163607
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
powder
mp
265 °C (dec.) (lit.)
functional group
carboxylic acid
ether
ketone
SMILES string
OC(=O)C1=CC(=O)C=C(O1)C(O)=O
InChI
1S/C7H4O6/c8-3-1-4(6(9)10)13-5(2-3)7(11)12/h1-2H,(H,9,10)(H,11,12)
InChI key
PBAYDYUZOSNJGU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Chelidonic acid (CA) is a γ-pyrone. It is reported as constituent of the rhizome of Chelidonium majus L. It has many pharmacological effects, such as mild analgesic and antimicrobial effects. Therapeutic potential of CA for the treatment of intestinal inflammation has been investigated. CA is reported as inhibitor of the rat brain glutamate decarboxylase. Biosynthesis of CA in cell suspension cultures of Leucojum aestivum has been studied.
Application
Chelidonic acid is suitable for use a in homogeneous preparation of native dihydrodipicolinate synthase from pea. It may be used as endocyclic oxygen-containing ligand in the synthesis of aqua[bis(2-pyridylmethyl)amine][chelidonato(1.5-)]-copper(II) chelidonate(0.5-) monohydrate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aqua [bis (2-pyridylmethyl) amine][chelidonato (1.5-)] copper (II) chelidonate (0.5-) monohydrate.
Fainerman-Melnikova M, et al.
Acta Crystallographica Section E, Structure Reports Online, 62(12), m3582-m3584 (2006)
C Dereppe et al.
Plant physiology, 98(3), 813-821 (1992-03-01)
Dihydrodipicolinate synthase (EC 4.2.1.52), the first enzyme unique to lysine biosynthesis in bacteria and higher plants, has been purified to homogeneity from etiolated pea (Pisum sativum) seedlings using a combination of conventional and affinity chromatographic steps. This is the first
Mohammad Ghadermazi et al.
Acta crystallographica. Section C, Crystal structure communications, 67(Pt 4), o134-o138 (2011-04-07)
Two related proton-transfer compounds, namely piperazine-1,4-diium 4-oxo-4H-pyran-2,6-dicarboxylate monohydrate, C(4)H(12)N(2)(2+)·C(7)H(2)O(6)(2-)·H(2)O or (pipzH(2))(cdo)·H(2)O, (I), and piperazine-1,4-diium bis(6-carboxy-4-oxo-4H-pyran-2-carboxylate), C(4)H(12)N(2)(2+)·2C(7)H(3)O(6)(-) or (pipzH(2))(cdoH)(2), (II), were obtained by the reaction of 4-oxo-4H-pyran-2,6-dicarboxylic acid (chelidonic acid, cdoH(2)) and piperazine (pipz). In (I), both carboxyl H atoms of
Dae-Seung Kim et al.
Biological & pharmaceutical bulletin, 35(5), 666-671 (2012-06-13)
Chelidonic acid (CA), a constituent of Chelidonium majus L., has many pharmacological effects, including mild analgesic and antimicrobial effects. However, the effects of CA on intestinal inflammation and the molecular mechanisms responsible are poorly understood. The aim of this study
Shen Z-W et al.
Phytochemistry, 57(1), 33-42 (2001-05-05)
The biosynthesis of chelidonic acid was studied in cell suspension cultures of Leucojum aestivum. Cell cultures were supplied with [U-13C]glucose, [l-13C]glucose or [U-13Cs]ribose/ribulose in standard medium containing unlabeled glucose. 13C labeling patterns of amino acids obtained by hydrolysis of biomass
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service