375276
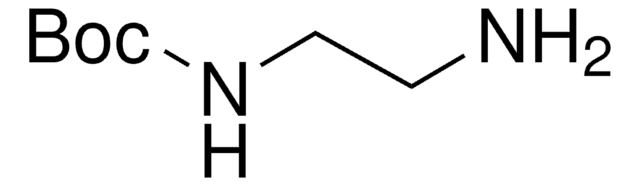
Di-tert-butyl-iminodicarboxylate
≥96%
Synonym(s):
N-Boc-tert-butylcarbamate, tert-Butyl iminodicarboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[(CH3)3COCO]2NH
CAS Number:
Molecular Weight:
217.26
Beilstein:
1911172
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥96%
mp
114-117 °C (lit.)
functional group
amine
SMILES string
CC(C)(C)OC(=O)NC(=O)OC(C)(C)C
InChI
1S/C10H19NO4/c1-9(2,3)14-7(12)11-8(13)15-10(4,5)6/h1-6H3,(H,11,12,13)
InChI key
XCAQIUOFDMREBA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Di-tert-butyl-iminodicarboxylate may be used in the preparation of:
- trans-diamino-2-butene and cis-1,4-diamino-2-butene
- di-tert-butyl N-3-butenyliminodicarboxylate
- N,N-di-tert-butyl[(2-fluoro-4-nitro)benzylamino]dicarboxylate
- C1-C20 and C21-C40 fragments of tetrafibricin
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Venugopal Gudipati et al.
Tetrahedron letters, 52(17), 2254-2257 (2011-05-24)
Efficient syntheses of suitably functionalized top and bottom fragments of tetrafibricin are described. The bottom fragment is prepared by two consecutive Kocienski-Julia couplings, while the top fragment synthesis features a dithiane alkylation and a Horner-Wadsworth-Emmons reaction.
K Kashiwagi et al.
The Journal of biological chemistry, 275(46), 36007-36012 (2000-08-31)
The PotE protein can catalyze both uptake and excretion of putrescine. The K(m) values of putrescine for uptake and excretion are 1.8 and 73 microm, respectively. Uptake of putrescine is dependent on the membrane potential, whereas excretion involves putrescine-ornithine antiporter
A convenient and efficient synthesis of (< i> S</i>)-lysine and (< i> S</i>)-arginine homologues via olefin cross-metathesis.
Boyle TP, et al.
Tetrahedron, 61(30), 7271-7276 (2005)
Young Ah Kim et al.
Biochemical pharmacology, 73(10), 1558-1572 (2007-02-20)
Toxoplasma gondii is an opportunistic pathogen responsible for toxoplasmosis. T. gondii is a purine auxotroph incapable of de novo purine biosynthesis and depends on salvage pathways for its purine requirements. Adenosine kinase (EC.2.7.1.20) is the major enzyme in the salvage
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service