371416
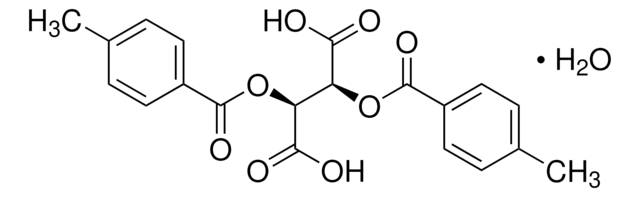
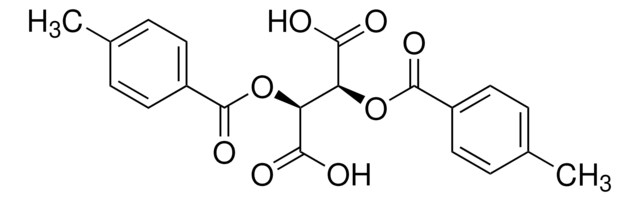
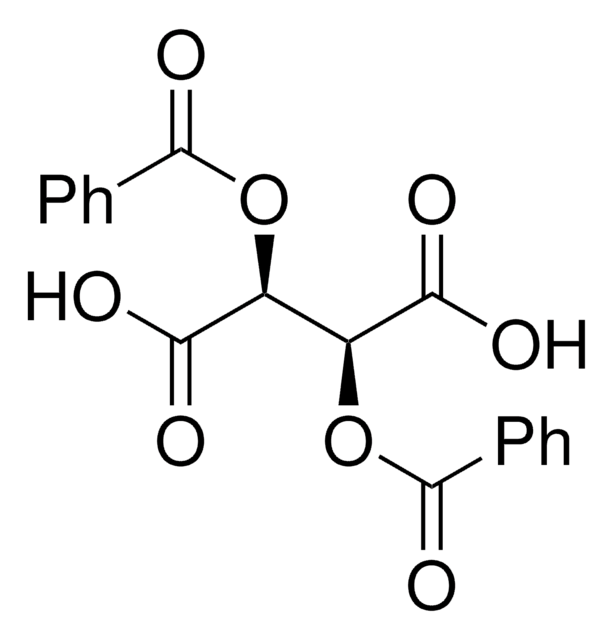
(−)-O,O′-Di-p-toluoyl-L-tartaric acid
97%
Synonym(s):
L-(-)-DTTA, Di-p-toluyl-L-tartaric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[CH3C6H4CO2CH(CO2H)-]2
CAS Number:
Molecular Weight:
386.35
Beilstein:
2022481
EC Number:
MDL number:
UNSPSC Code:
12352106
eCl@ss:
39021705
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
optical activity
[α]20/D −138°, c = 1 in ethanol
mp
169-171 °C (lit.)
SMILES string
Cc1ccc(cc1)C(=O)O[C@H]([C@@H](OC(=O)c2ccc(C)cc2)C(O)=O)C(O)=O
InChI
1S/C20H18O8/c1-11-3-7-13(8-4-11)19(25)27-15(17(21)22)16(18(23)24)28-20(26)14-9-5-12(2)6-10-14/h3-10,15-16H,1-2H3,(H,21,22)(H,23,24)/t15-,16-/m1/s1
InChI key
CMIBUZBMZCBCAT-HZPDHXFCSA-N
General description
(-)-O,O′-Di-p-toluoyl-L-tartaric acid is a O,O′-diacyl tartaric acid derivative that can be prepared by the resolution of its racemate in the presence of cinchonine.
Application
(-)-O,O′-Di-p-toluoyl-L-tartaric acid may be used as a chiral resolving agent for the resolution of the racemic bases to isolate the (-)-enantiomeric forms.
Resolving agent for racemic amines.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Highly efficient resolution of (?)-Tramadol with di-p-toluoyl-tartaric acid (DTTA).
Evans GR, et al.
Tetrahedron Asymmetry, 12(11) null
Synthetic Communications, 20, 3553-3553 (1990)
Tetrahedron Letters, 32, 7325-7325 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service