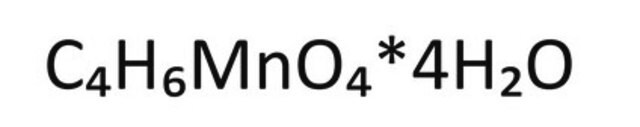330825
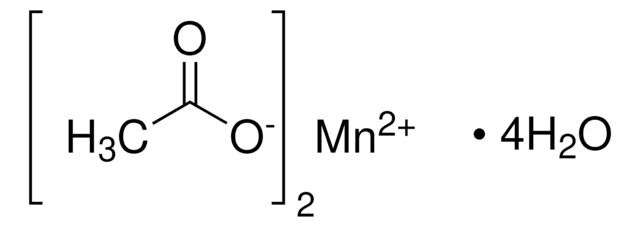
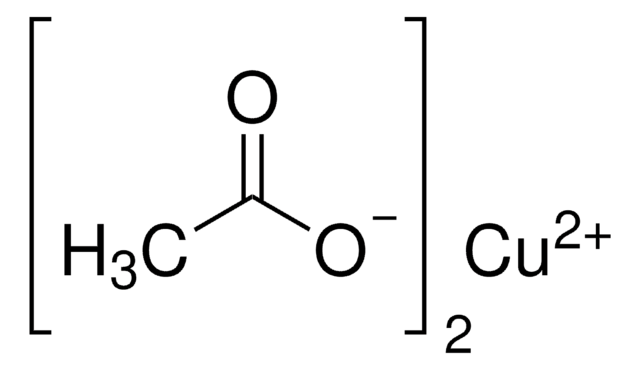
Manganese(II) acetate
98%
Synonym(s):
Diacetylmanganese, Manganous acetate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3CO2)2Mn
CAS Number:
Molecular Weight:
173.03
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
powder
reaction suitability
core: manganese
SMILES string
CC(=O)O[Mn]OC(C)=O
InChI
1S/2C2H4O2.Mn/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChI key
UOGMEBQRZBEZQT-UHFFFAOYSA-L
General description
Manganese (II) acetate is a crystalline solid that can be synthesized by reacting acetic acid withmanganese (II,III) oxide or manganese(II) carbonate. It is used as a sol-gel precursor to synthesize thin films and a critical precursor for the synthesis of cathode-active materials for rechargeable batteries.
Application
Manganese(II) acetate can be used:
- As a precursor to synthesize manganese oxide nanoparticles for various applications such as gas sensing using the solvent-free method.
- To fabricate electrodes for high-performance Li-ion batteries.
- Suitable for the fabrication of spinel/layered heterostructured cathode materials for high-capacity and high-rate Li-Ion batteries.
- As a starting material to prepare Mn-based catalysts.
- To fabricate pyridine manganese halide scintillators for X-ray imaging.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - STOT RE 2 Inhalation
Target Organs
Brain
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Eleonora Pargoletti et al.
Nanomaterials (Basel, Switzerland), 10(9) (2020-09-05)
One of the major drawbacks in Lithium-air batteries is the sluggish kinetics of the oxygen reduction reaction (ORR). In this context, better performances can be achieved by adopting a suitable electrocatalyst, such as MnO2. Herein, we tried to design nano-MnO2
Tingwei Zhang et al.
ChemSusChem, 11(16), 2730-2736 (2018-06-01)
The rational design of highly efficient and durable oxygen reduction reaction (ORR) catalysts is critical for the commercial application of fuel cells. Herein, three-dimensional graphene (3D-G) is synthesized by the template method, which used coal tar pitch as the carbon
Fangchun Han et al.
Chemistry, an Asian journal, 12(17), 2284-2290 (2017-08-02)
This work demonstrates a facile in situ synthesis of cobalt-manganese mixed sulfide (CoMn-S) nanocages on reduced graphene oxide (RGO) sheets by using a crystalline Co-Mn precursor as the sacrificial template. The CoMn-S/RGO hybrid was applied as the anode for Li-ion
Eesh Vaghela et al.
Physical chemistry chemical physics : PCCP, 19(7), 5163-5176 (2017-02-01)
In this communication, structural, microstructural, transport and magnetotransport properties are reported for La
Jia Yao et al.
Chemical science, 9(11), 2927-2933 (2018-05-08)
Reactive oxygen species (ROS)-induced oxidative stress is linked to various diseases, including cardiovascular disease and cancer. Though highly efficient natural ROS scavenging enzymes have been evolved, they are sensitive to environmental conditions and hard to mass-produce. Therefore, enormous efforts have
Articles
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service