All Photos(2)
About This Item
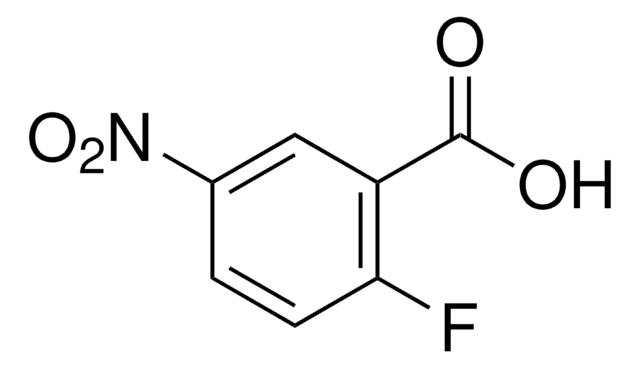
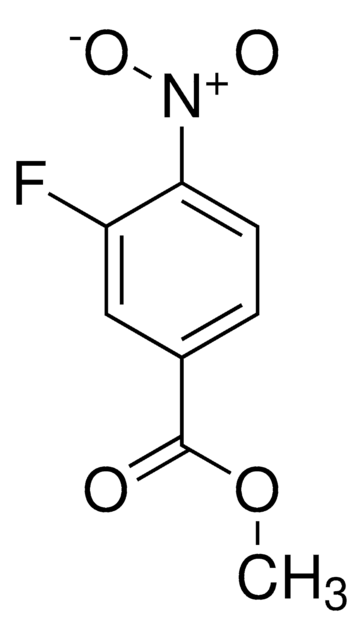
Linear Formula:
FC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
185.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
123-126 °C (lit.)
solubility
95% ethanol: soluble 50 mg/mL, clear, light yellow
functional group
carboxylic acid
fluoro
nitro
SMILES string
OC(=O)c1ccc(F)c(c1)[N+]([O-])=O
InChI
1S/C7H4FNO4/c8-5-2-1-4(7(10)11)3-6(5)9(12)13/h1-3H,(H,10,11)
InChI key
BOJWTAQWPVBIPG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Fluoro-3-nitrobenzoic acid was used:
- as starting reagent in the preparation of novel benzimidazoles having antimycobacterial activity
- in preparation of series of novel acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors containing benzimidazole core structure
- in preparation of bis(heterocyclic) skeletal precursors for the Pictet-Spengler reaction
- in solid-phase synthesis of trisubstituted [1,3,5]triazino[1,2-a]benzimidazole-2,4(3H,10H)-diones
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chih-Hau Chen et al.
Chemistry, an Asian journal, 6(6), 1557-1565 (2011-04-08)
A novel strategy for an unconventional Pictet-Spengler reaction has been developed for the regioselective cyclization of the imidazole ring system at the C2 position. The developed strategy was utilized to develop a diversity-oriented parallel synthesis for bis(heterocyclic) skeletal novel analogs
Gérard Klein et al.
Journal of combinatorial chemistry, 4(4), 345-351 (2002-07-09)
An efficient method for the solid-phase synthesis of trisubstituted [1,3,5]triazino[1,2-a]benzimidazole-2,4(3H,10H)-diones from resin-bound amino acids is described. N-acylation of the primary amine of a resin-bound amino acid with 4-fluoro-3-nitrobenzoic acid, followed by displacement of the fluoro group and reduction of the
Yeong Keng Yoon et al.
European journal of medicinal chemistry, 93, 614-624 (2014-07-06)
A total of 51 novel benzimidazoles were synthesized by a 4-step reaction starting from basic compound 4-fluoro-3-nitrobenzoic acid under relatively mild reaction conditions. The structure of the novel benzimidazoles was confirmed by mass spectra as well as (1)H NMR spectroscopic
Yeong Keng Yoon et al.
Bioorganic chemistry, 49, 33-39 (2013-07-28)
Two series of novel acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors containing benzimidazole core structure were synthesized by a four-step reaction pathway starting from 4-fluoro-3-nitrobenzoic acid as the basic compound. The structure of the novel benzimidazoles was characterized and confirmed by
Wen-Bing Yeh et al.
Combinatorial chemistry & high throughput screening, 7(3), 251-255 (2004-05-12)
Liquid phasel synthesis of biheterocyclic benzimidazoles by controlled microwave irradiation was investigated. Polymer immobilized o-phenylenediamines was synthesized under microwave irradiation. The resulting PEG bound diamines was N-acylated with 4-fluoro-3-nitrobenzoic acid selectively in primary aromatic amino moiety. Nucleophilic aromatic substitution of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service