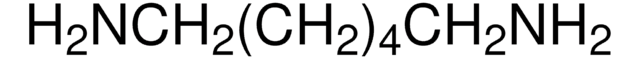247731
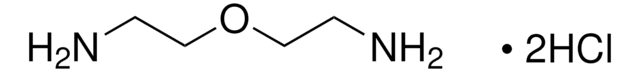
Hexamethylenediamine dihydrochloride
99%
Synonym(s):
1,6-Hexanediamine dihydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2N(CH2)6NH2 · 2HCl
CAS Number:
Molecular Weight:
189.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
256-257 °C (lit.)
solubility
water: freely soluble
functional group
amine
SMILES string
Cl.Cl.NCCCCCCN
InChI
1S/C6H16N2.2ClH/c7-5-3-1-2-4-6-8;;/h1-8H2;2*1H
InChI key
XMVQMBLTFKAIOX-UHFFFAOYSA-N
General description
Toxicity of hexamethylenediamine dihydrochloride has been investigated. Hexamethylenediamine dihydrochloride is also known as 1,6-diaminohexane dihydrochloride, 1,6-hexamethylenediamine dihydrochloride, 1,6- hexylenediamine dihydrochloride or 1,6-diamino-n-hexane dihydrochloride. Hexamethylenediamine dihydrochloride on fusion of 1:6-di-(N3-cyano-N1-guanidino)-hexane yields polymeric diguanides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Charles Hebert
Toxicity report series, 24, 1-D8-1-D8 (1993-03-01)
1,6-Hexanediamine (HDA) is an aliphatic amine that is produced in large volumes in the United States. HDA is widely used as a corrosion inhibitor in lubricants and as an intermediate in the industrial synthesis of paints, resins, inks, and textiles.
850. Bisdiguanides having antibacterial activity.
Rose FL andSwain G.
Journal of the Chemical Society, 4422-4425 (1956)
Richard F G Fröhlich et al.
Carbohydrate research, 346(12), 1592-1598 (2011-06-08)
Two simple and reliably accessible intermediates, N-carboxypentyl- and N-aminohexyl-1-deoxy-D-galactonojirimycin were employed for the synthesis of a set of terminally N-dansyl substituted derivatives. Reaction of the terminal carboxylic acid of N-carboxypentyl-1-deoxy-D-galactonojirimycin with N-dansyl-1,6-diaminohexane provided the chain-extended fluorescent derivative. Employing bis(6-dansylaminohexyl)amine, the
Zhiming Zhang et al.
Inorganic chemistry, 47(17), 7615-7622 (2008-08-06)
The reaction between K 12[H 2P 2W 12O 48] and CuCl 2 in a NaCl aqueous solution assisted with organoamines (1,2-ethylenediamine (en), 1,6-hexamethylene diamine (hn), or both) leads to the isolation of three compounds: K 4Na 10[alpha 1-CuP 2W 17O
Wei-Chiang Chen et al.
ACS applied materials & interfaces, 1(8), 1821-1826 (2010-04-02)
Solvent microenvironments are formed around individual single-walled carbon nanotubes (SWNTs) by mixing SWNT suspensions with water-immiscible organic solvents. These microenvironments are used to encapsulate the SWNTs with the monomer sebacoyl chloride. Hexamethylene diamine is then injected into the aqueous phase
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service