220159
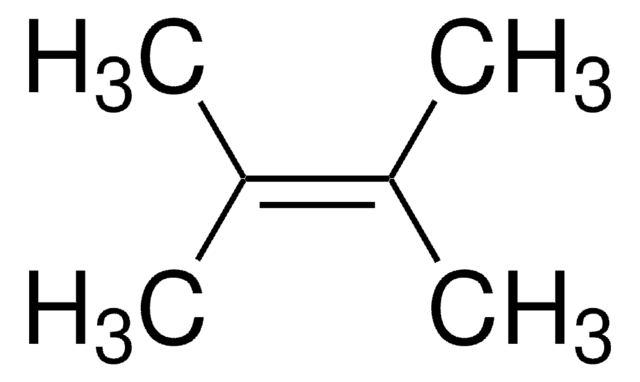
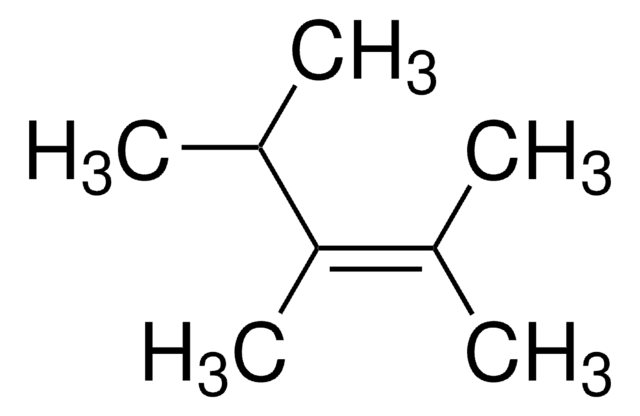
2,3-Dimethyl-2-butene
≥99%
Synonym(s):
Tetramethylethylene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2C=C(CH3)2
CAS Number:
Molecular Weight:
84.16
Beilstein:
1361357
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
215 mmHg ( 37.7 °C)
Assay
≥99%
form
liquid
autoignition temp.
754 °F
refractive index
n20/D 1.412 (lit.)
bp
73 °C (lit.)
mp
−75 °C (lit.)
density
0.708 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C\C(C)=C(\C)C
InChI
1S/C6H12/c1-5(2)6(3)4/h1-4H3
InChI key
WGLLSSPDPJPLOR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2,3-Dimethyl-2-butene undergoes ozonolysis in dark to yield hydroxyl radical. Reaction of ozone with 2,3-dimethyl 2-butene (DMB) has been investigated using a flow-tube interfaced to UV photoelectron spectrometer. DMB forms adduct with thianthrene cation radical tetrafluoroborate at 0°C and -15°C.
Application
2,3-Dimethyl-2-butene was employed as substrate in photoinduced molecular transformations involving 2-hydroxy-1,4-naphthoquinones.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
17.6 °F - closed cup
Flash Point(C)
-8 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The Journal of Organic Chemistry, 58, 4614-4614 (1993)
Bing-Jun Zhao et al.
The Journal of organic chemistry, 71(10), 3737-3742 (2006-05-06)
Thianthrene cation radical tetrafluoroborate (Th*+ BF4-) has been found to add to 2,3-dimethyl-2-butene (DMB) at 0 degrees C and -15 degrees C. The adduct, 2,3-dimethyl-2,3-(5,10-thianthreniumdiyl)butane ditetrafluoroborate (12), was isolated at -15 degrees C, and its 1H NMR spectrum was recorded
Maryline Pflieger et al.
Environmental science & technology, 47(12), 6239-6246 (2013-05-15)
In order to investigate the heterogeneous oxidation kinetics of the herbicide terbuthylazine (TERB), a stable and reproducible generation system of "dark" hydroxyl radical in the gas phase was developed and optimized using a PTR-MS. TERB was adsorbed on silica particles
L R Pohl et al.
Biochemical and biophysical research communications, 117(2), 367-371 (1983-12-16)
Although indirect evidence has suggested that liver microsomal cytochrome P-450 can reductively dehalogenate several compounds to carbene metabolites, there has been no direct proof for the formation of these reactive species. We report in this paper that carbenes can be
R Tolando et al.
Xenobiotica; the fate of foreign compounds in biological systems, 26(4), 425-435 (1996-04-01)
1. During anaerobic reductive incubation of liver microsomes, from either the pyridine- or phenobarbital-treated rat, with 1,1-dichloro-1-fluoroethane (HCFC-141b) in the presence of a NADPH-regenerating system, a time- and dose-dependent formation of reactive metabolites was detected as indicated by a depletion
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service