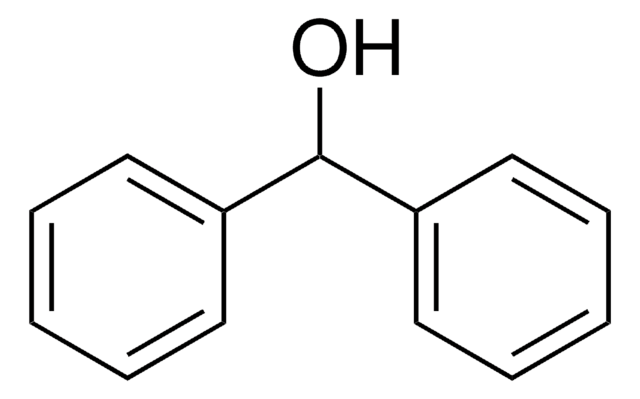
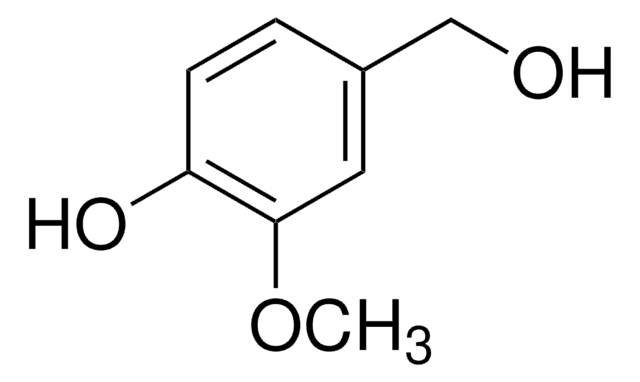
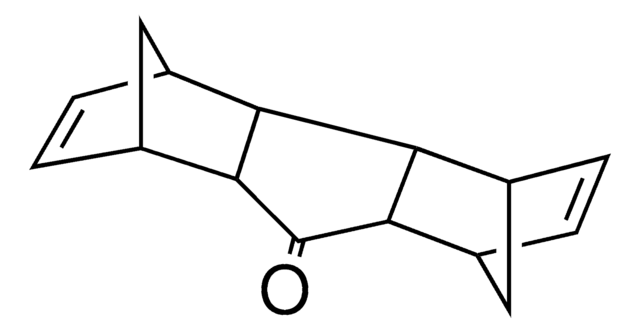
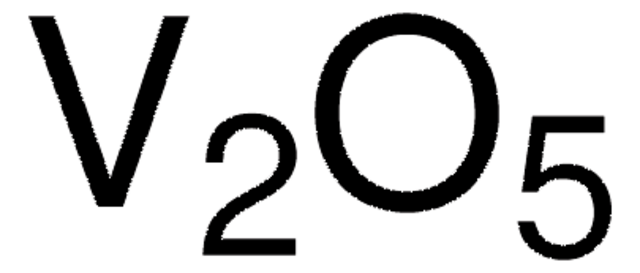213462
Sodium borohydride
ReagentPlus®, 99%
Synonym(s):
Sodium tetrahydridoborate
About This Item
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
form
powder
reaction suitability
reagent type: reductant
mp
>300 °C (dec.) (lit.)
SMILES string
[BH4-].[Na+]
InChI
1S/BH4.Na/h1H4;/q-1;+1
InChI key
YOQDYZUWIQVZSF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Synthesis of cobalt (II, III) oxide (Co3O4) from cobalt chloride (CoCl2) via a co-reduction process.
- Solvent-free reduction of aldehydes and ketones to alcohols in the presence of solid acid as an activator.
- Reductive amination of carbonyl compounds to secondary amines in the presence of silica-gel-supported sulfuric acid as a catalyst.
- Raney nickel-catalyzed reduction of aromatic nitro compounds to aromatic amines.
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Repr. 1B - Skin Corr. 1B - Water-react 1
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Solvothermal synthesis is a method for preparing a variety of materials such as metals, semiconductors, ceramics, and polymers.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service