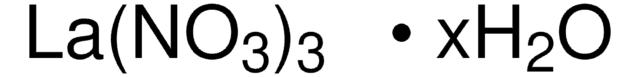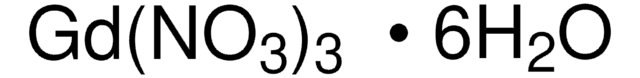203548
Lanthanum(III) nitrate hexahydrate
99.999% trace metals basis
Synonym(s):
Nitric acid, lanthanum (III) salt, hexahydrate
About This Item
Recommended Products
Assay
99.999% trace metals basis
form
solid
reaction suitability
reagent type: catalyst
core: lanthanum
impurities
≤15.0 ppm Trace Rare Earth Analysis
mp
65-68 °C
SMILES string
[La+3].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/La.3NO3.6H2O/c;3*2-1(3)4;;;;;;/h;;;;6*1H2/q+3;3*-1;;;;;;
InChI key
GJKFIJKSBFYMQK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- α-aminonitriles by a one-pot three-component coupling of the carbonyl compound, amine, and TMSCN.
- Acetals and geminal diacetates (acylals)from aldehydes.
- Bis(indolyl) methanes under solvent‐free conditions.
Features and Benefits
- Thermal stability
- Low toxicity
- Mild reaction condition
- Chemoselectivity
- Low cost
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Ox. Sol. 2
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 2
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Spectral conversion for solar cells is an emerging concept in the field of photovoltaics, and it has the potential to increase significantly the efficiency of solar cells. Lanthanide ions are ideal candidates for spectral conversion, due to their high luminescence efficiencies and rich energy level structure that allows for great flexibility in the upconversion and downconversion of photons in a wide spectral region (NIR-VIS-UV).
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service