All Photos(2)
About This Item
Empirical Formula (Hill Notation):
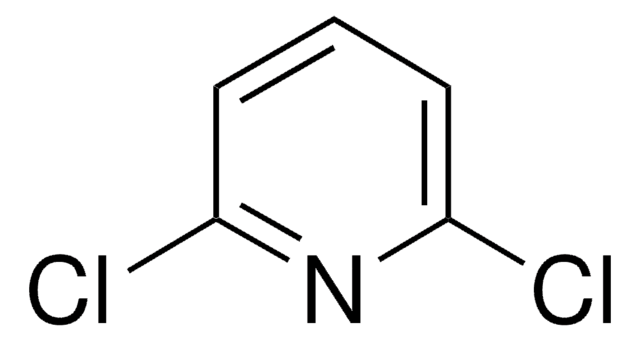
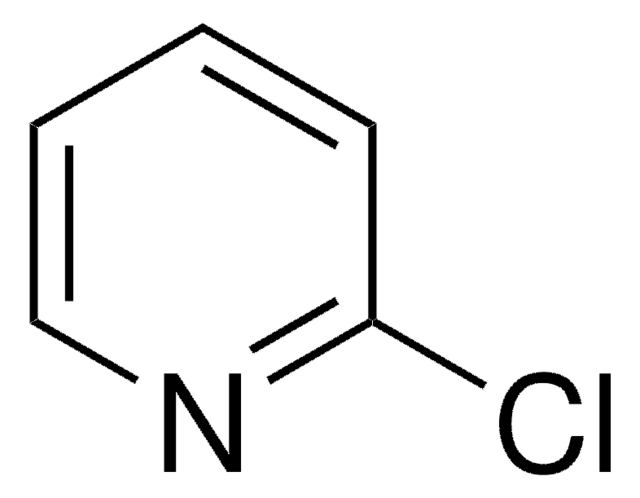
C5H3Cl2N
CAS Number:
Molecular Weight:
147.99
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
59-62 °C (lit.)
functional group
chloro
SMILES string
Clc1ccc(Cl)nc1
InChI
1S/C5H3Cl2N/c6-4-1-2-5(7)8-3-4/h1-3H
InChI key
GCTFDMFLLBCLPF-UHFFFAOYSA-N
General description
2,5-Dichloropyridine undergoes cross-coupling reaction with arylboronic acids in the presence of [1,4-bis-(diphenylphosphine)butane]palladium (II) dichloride as catalyst.
Application
2,5-Dichloropyridine was used in the synthesis of 6-halo-pyridin-3-yl boronic acids and esters.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Coupling of heteroaryl chlorides with arylboronic acids in the presence of [1, 4-bis-(diphenylphosphine) butane] palladium (II) dichloride.
Mitchell MB and Wallbank PJ.
Tetrahedron Letters, 32(20), 2273-2276 (1991)
Synthesis of novel halopyridinylboronic acids and esters. Part 1: 6-Halopyridin-3-yl-boronic acids and esters.
Bouillon A, et al.
Tetrahedron, 58(14), 2885-2890 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service