All Photos(1)
About This Item
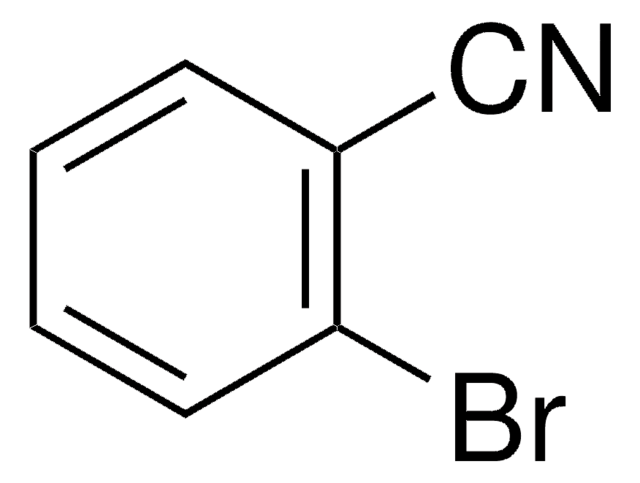
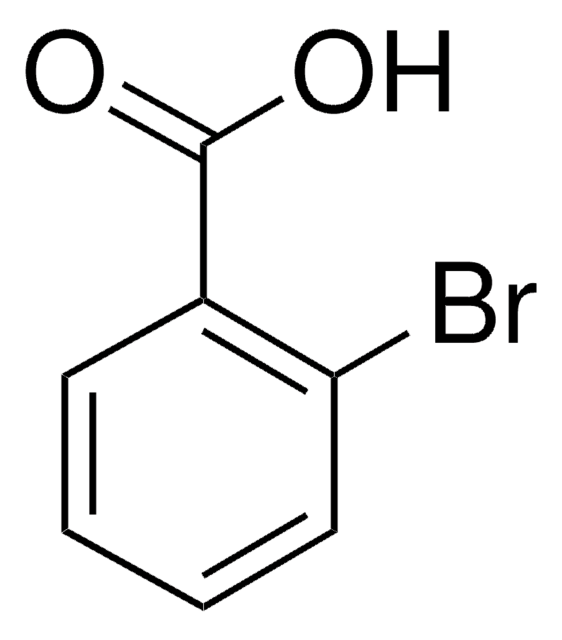
Linear Formula:
BrC6H4CONH2
CAS Number:
Molecular Weight:
200.03
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
160-162 °C (lit.)
functional group
amide
bromo
SMILES string
NC(=O)c1ccccc1Br
InChI
1S/C7H6BrNO/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,(H2,9,10)
InChI key
NHNAEZDWNCRWRW-UHFFFAOYSA-N
Related Categories
Application
2-Bromobenzamide has been used in:
- microwave assisted one-pot synthesis of substituted 3-(phenylmethylene)isoindolin-1-ones
- palladium-catalyzed synthesis of phenanthridinones
- synthesis of new water-soluble iminophosphorane ligand
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Monica Carreira et al.
Organometallics, 31(16), 5772-5781 (2012-10-16)
The synthesis and characterization of a new water-soluble iminophosphorane ligand TPA=N-C(O)-2BrC(6)H(4) (C,N-IM; TPA = 1,3,5-triaza-7-phosphaadamantane) 1 is reported. Oxidative addition of 1 to Pd(2)(dba)(3) affords the orthopalladated dimer [Pd(μ-Br){C(6)H(4)(C(O)N=TPA-kC,N)-2}](2) (2) as a mixture of cis and trans isomers (1:1 molar
Chun Lu et al.
The Journal of organic chemistry, 77(19), 8648-8656 (2012-09-28)
The palladium-catalyzed annulation of arynes by substituted o-halobenzamides produces N-substituted phenanthridinones in good yields. This methodology provides this important heterocyclic ring system in a single step by simultaneous C-C and C-N bond formation, under relatively mild reaction conditions, and tolerates
Microwave assisted copper-free Sonogashira coupling/5-exo-dig cycloisomerization domino reaction: access to 3-(phenylmethylene) isoindolin-1-ones and related heterocycles.
Hellal M and Cuny GD.
Tetrahedron Letters, 52(42), 5508-5511 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service