180823
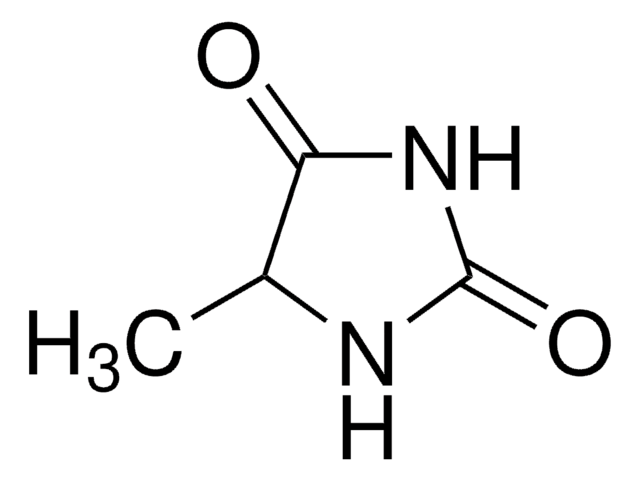
5-Methyl-5-phenylhydantoin
99%
Synonym(s):
5-Methyl-5-phenylimidazolidine-2,4-dione, NSC 14839
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H10N2O2
CAS Number:
Molecular Weight:
190.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
199-201 °C (lit.)
separation technique
chiral
SMILES string
CC1(NC(=O)NC1=O)c2ccccc2
InChI
1S/C10H10N2O2/c1-10(7-5-3-2-4-6-7)8(13)11-9(14)12-10/h2-6H,1H3,(H2,11,12,13,14)
InChI key
JNGWGQUYLVSFND-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for synthesis of:
Beta-amino alcohols as inhibitors of anti-tubercular target N-acetyltransferase
Chlorohydantoins
Specific MMP inhibitors
Beta-amino alcohols as inhibitors of anti-tubercular target N-acetyltransferase
Chlorohydantoins
Specific MMP inhibitors
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Daniel C Whitehead et al.
Tetrahedron letters, 50(6), 656-658 (2010-02-17)
A simple and efficient methodology for the preparation of N-chlorinated hydantoins is presented. These versatile chlorenium sources were isolated in high yield after a simple recrystallization. Among the ten examples are the first chiral N-chlorohydantoins.
Pirkle WH and Gan KZ.
Journal of Chromatography A, 790(1), 65-71 (1997)
Elizabeth Fullam et al.
Bioorganic & medicinal chemistry letters, 21(4), 1185-1190 (2011-01-22)
The synthesis and inhibitory potencies of a novel series of β-amino alcohols, based on the hit-compound 3-[3'-(4''-cyclopent-2'''-en-1'''-ylphenoxy)-2'-hydroxypropyl]-5,5 dimethylimidazolidine-2,4-dione as specific inhibitors of mycobacterial N-acetyltransferase (NAT) enzymes are reported. Effects of synthesised compounds on growth of Mycobacterium tuberculosis have been determined.
Adriana Bakalova et al.
European journal of medicinal chemistry, 38(6), 627-632 (2003-07-02)
The interaction of cis-dichlorodiaminplatinum(II) (cis-DDP) with 2,4-imidazolidenedione-5-methyl-5-phenyl was studied. The method of preparation of the new Pt(II) complex consisted in precipitation of chloride ions from cis-DDP via a diaqua complex and reaction with the ligand in water-organic media. On the
B Chankvetadz et al.
Journal of pharmaceutical and biomedical analysis, 15(9-10), 1577-1584 (1997-06-01)
The techniques for a dynamic and permanent reversal of the electroosmotic flow (EOF) were used for the reversal of the enantiomer migration order (EMO) of neutral and cationic analytes in chiral capillary electrophoresis (CE). Native beta-Cd and an anionic CD
Chromatograms
application for HPLCapplication for HPLCapplication for HPLCapplication for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service