168637
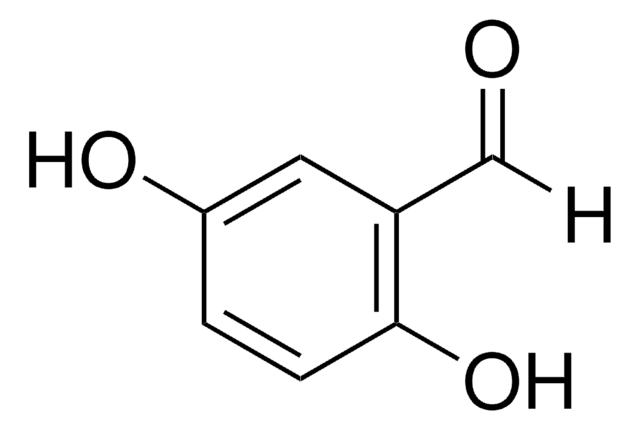
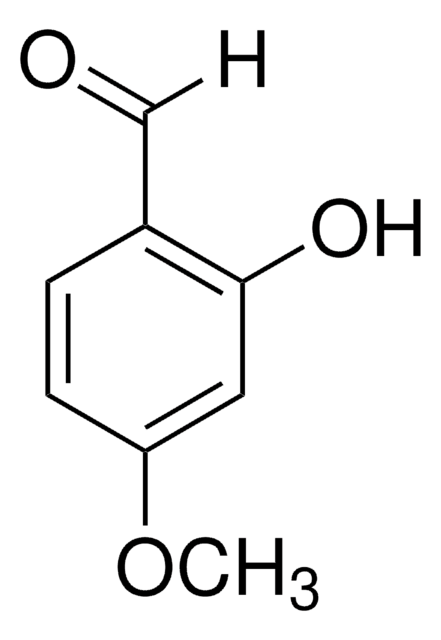
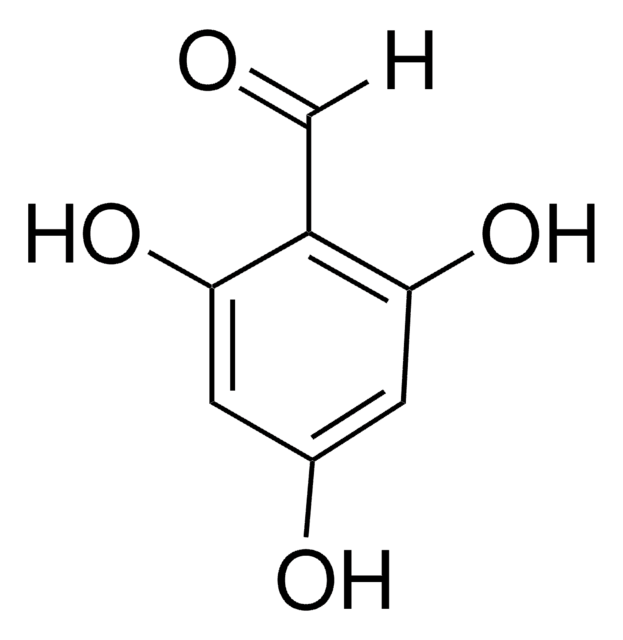
2,4-Dihydroxybenzaldehyde
98%
Synonym(s):
β-Resorcylaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(HO)2C6H3CHO
CAS Number:
Molecular Weight:
138.12
Beilstein:
878548
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
bp
220-228 °C/22 mmHg (lit.)
mp
135-137 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ccc(O)cc1O
InChI
1S/C7H6O3/c8-4-5-1-2-6(9)3-7(5)10/h1-4,9-10H
InChI key
IUNJCFABHJZSKB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4-Dihydroxybenzaldehyde undergoes regioselective mono-benzylation reaction under extremely mild basic conditions. It undergoes condensation reaction with isonicotinic acid hydrazide in methanol to yield 2,4-dihydroxybenzaldehyde isonicotinoyl hydrazone, a new fluorescent reagent.
2,4-Dihydroxybenzaldehyde is a potential building block for bioactive compounds, specialty chemicals and dyes.
2,4-Dihydroxybenzaldehyde is a potential building block for bioactive compounds, specialty chemicals and dyes.
Application
2,4-Dihydroxybenzaldehyde was used in two-step synthesis of ethyl 3,5-dibromo-2,4-dihydroxycinnamate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The regioselective 4-benzylation of 2, 4-dihydroxybenzaldehyde.
Mendelson WL, et al.
Synthetic Communications, 26(3), 593-601 (1996)
Two-photon uncaging with the efficient 3,5-dibromo-2,4-dihydroxycinnamic caging group.
Nathalie Gagey et al.
Angewandte Chemie (International ed. in English), 46(14), 2467-2469 (2007-02-21)
Direct and derivative spectrophotometric determination of zinc with 2, 4-dihydroxybenzaldehyde isonicotinoyl hydrazone in potable water and pharmaceutical samples.
Sivaramaiah S and Reddy PR.
Journal of Analytical Chemistry, 60(9), 828-832 (2005)
Fabien Vincent et al.
Bioorganic & medicinal chemistry letters, 19(23), 6793-6796 (2009-10-24)
The screening of known medicinal agents against new biological targets has been shown to be a valuable approach for revealing new pharmacology of marketed compounds. Recently, carbamate, urea and ketone inhibitors of fatty acid amide hydrolase (FAAH) have been described
Chao-Bin Xue et al.
Bioorganic & medicinal chemistry, 15(5), 2006-2015 (2007-01-30)
Phenoloxidase (PO), also known as tyrosinase, is a key enzyme in insect development, responsible for catalyzing the hydroxylation of tyrosine into o-diphenols and the oxidation of o-diphenols into o-quinones. Inhibition of PO may provide a basis for novel environmentally friendly
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 168637-25G | 4061838749963 |
| 168637-100G | 4061838749956 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service