All Photos(1)
About This Item
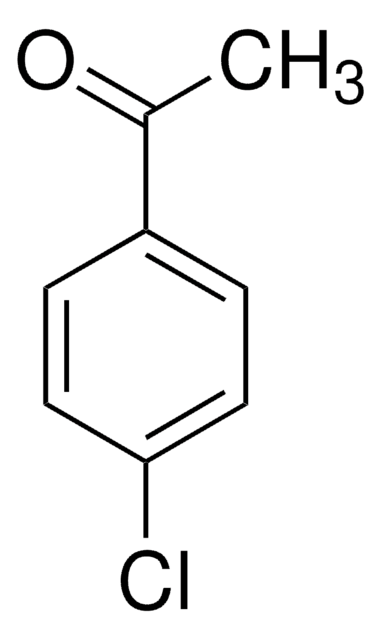
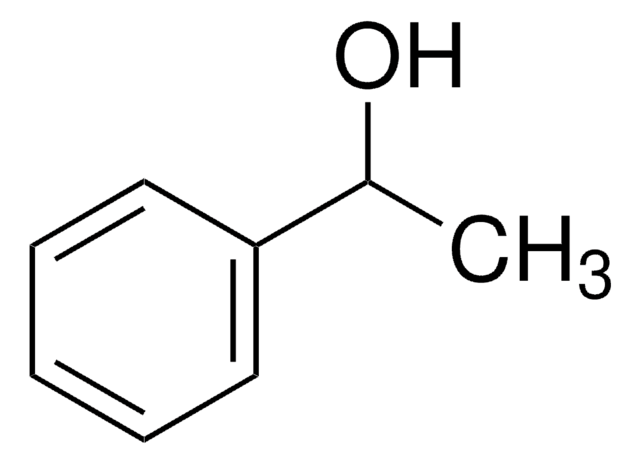
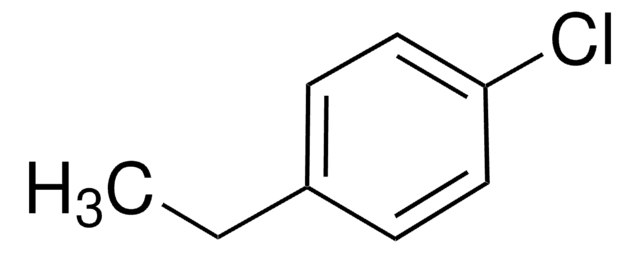
Linear Formula:
ClC6H4CH(CH3)OH
CAS Number:
Molecular Weight:
156.61
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.541 (lit.)
bp
119 °C/10 mmHg (lit.)
density
1.171 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
CC(O)c1ccc(Cl)cc1
InChI
1S/C8H9ClO/c1-6(10)7-2-4-8(9)5-3-7/h2-6,10H,1H3
InChI key
MVOSNPUNXINWAD-UHFFFAOYSA-N
Application
1-(4-Chlorophenyl)ethanol was used to study transfer dehydrogenation of various alcohols over heterogeneous palladium catalysts using olefins as hydrogen acceptors.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Selective transfer dehydrogenation of aromatic alcohols on supported palladium.
Keresszegi C, et al.
New. J. Chem., 25(9), 1163-1167 (2001)
Xiao-Hong Chen et al.
BMC biotechnology, 11, 110-110 (2011-11-22)
Chiral alcohols are widely used in the synthesis of chiral pharmaceuticals, flavors and functional materials and appropriate whole-cell biocatalysts offer a highly enantioselective, minimally polluting route to these valuable compounds. The recently isolated strain Acetobacter sp. CCTCC M209061 showed exclusive
Xiao-Hong Chen et al.
PloS one, 9(4), e94543-e94543 (2014-04-18)
A novel carbonyl reductase (AcCR) catalyzing the asymmetric reduction of ketones to enantiopure alcohols with anti-Prelog stereoselectivity was found in Acetobacter sp. CCTCC M209061 and enriched 27.5-fold with an overall yield of 0.4% by purification. The enzyme showed a homotetrameric
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service