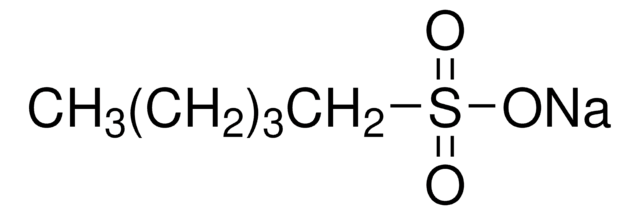106437
Sodium 1-dodecanesulfonate
ReagentPlus®, ≥99%
Synonym(s):
1-Dodecanesulfonic acid sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3(CH2)11SO3Na
CAS Number:
Molecular Weight:
272.38
Beilstein:
3919536
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
≥99%
mp
>300 °C (lit.)
solubility
H2O: 25 mg/mL
functional group
sulfonic acid
SMILES string
[Na+]
InChI
1S/C12H26O3S.Na/c1-2-3-4-5-6-7-8-9-10-11-12-16(13,14)15;/h2-12H2,1H3,(H,13,14,15);/q;+1/p-1
InChI key
DAJSVUQLFFJUSX-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Sodium 1-dodecanesulfonate is an ion-pairing reagent and increases the retention of thiabendazole on the HPLC column during determination of thiabendazole in citrus fruit and banana. It is a surfactant standard.
Application
Sodium 1-dodecanesulfonate may be used in synthesis of metal oxide-graphene nanocomposites.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chengcheng Yu et al.
Polymers, 11(5) (2019-05-11)
In this work, the effect of doubly functionalized montmorillonite (MMT) on the structure, morphology, thermal, and tribological characteristics of the resulting polystyrene (PS) nanocomposites was investigated. The modification of the MMT was performed using a cationic surfactant and an anionic
Donghai Wang et al.
ACS nano, 4(3), 1587-1595 (2010-02-27)
Surfactant or polymer directed self-assembly has been widely investigated to prepare nanostructured metal oxides, semiconductors, and polymers, but this approach is mostly limited to two-phase materials, organic/inorganic hybrids, and nanoparticle or polymer-based nanocomposites. Self-assembled nanostructures from more complex, multiscale, and
Y Ito et al.
Journal of chromatography. A, 810(1-2), 81-87 (1998-08-06)
A simple, rapid and reproducible analytical method for thiabendazole (TBZ) and imazalil (IMA) in citrus fruit and banana has been developed. The method involves the use of an ion-exchange cartridge for sample clean-up followed by ion-pair high-performance liquid chromatography with
Toshihide Tsukatani et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 22(2), 199-200 (2006-03-04)
The tetraoctylammonium cation forms water-immiscible room temperature ionic liquids with dodecylsulfate and dodecylbenzenesulfonate anions. The ionic liquids are halogen-free and can be considered environmentally friendly solvents. At 25 degrees C, the solubilities of water in tetraoctylammonium dodecylsulfate and tetraoctylammonium dodecylbenzenesulfonate
Manuel Mora et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 78(3), 989-995 (2011-01-14)
An Mg/Al layered double hydroxide (LDH) containing carbonate ion in its interlayer region was examined by medium infrared (MIR) and near infrared reflectance spectroscopy (NIRS). The MIR and NIR spectroscopy techniques was also used to study two organo-hybrid LDHs containing
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide 95%](/deepweb/assets/sigmaaldrich/product/structures/217/219/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da/640/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da.png)