T200
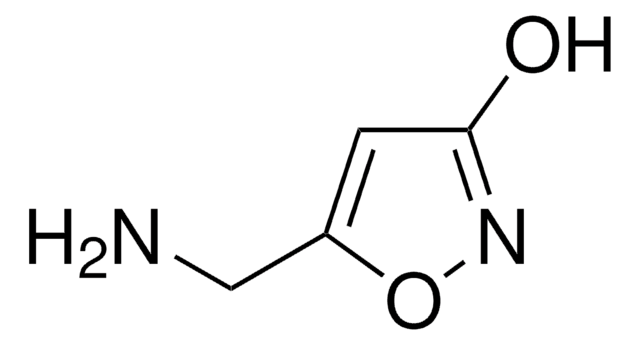
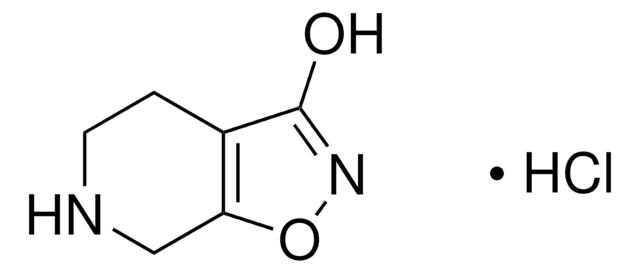
(1,2,5,6-Tetrahydropyridin-4-yl)methylphosphinic acid hydrate
≥97% (HPLC), solid
Synonym(s):
TPMPA hydrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H12NO2P · xH2O
CAS Number:
Molecular Weight:
161.14 (anhydrous basis)
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥97% (HPLC)
form
solid
color
white to off-white
solubility
H2O: 16 mg/mL
DMSO: insoluble
SMILES string
[H]O[H].CP([O-])(=O)C1=CC[NH2+]CC1
InChI
1S/C6H12NO2P.H2O/c1-10(8,9)6-2-4-7-5-3-6;/h2,7H,3-5H2,1H3,(H,8,9);1H2
InChI key
GXVWAQPRAQORGS-UHFFFAOYSA-N
Application
(1,2,5,6-Tetrahydropyridin-4-yl)methylphosphinic acid hydrate has also been used as γ-aminobutyric acid C (GABAc) blocker in retinal ganglion cells.
TPMA has been used as a GABAC receptor antagonist to study the role of inner retinal inhibition in midget ganglion cells. TPMPA has also been used to study the role of GABAC receptor antagonists in suppressing orientation selectivity in ganglion cells.
Biochem/physiol Actions
TPMPA is a hybrid of isoguvacine and 3-APMPA designed to retain affinity for GABAC receptors but not to interact with GABAA or GABAB receptors. Electrical assays show that TPMPA is a competitive antagonist of cloned human mu 1 GABAC receptors expressed in Xenopus laevis oocytes (Kb approx. 2 μM). TPMPA is >100-fold weaker as an inhibitor of rat brain GABAA receptors expressed in oocytes (Kb approx. 320 μM) and has only weak agonist activity on GABAB receptors assayed in rat hippocampal slices (EC50 approx. 500 μM). TPMPA may be used to investigate GABAC receptor function in the outer retina and in any other areas of the nervous system in which these types of receptor are present.
Features and Benefits
This compound is a featured product for Neuroscience research. Click here to discover more featured Neuroscience products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the GABAC Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Preparation Note
(1,2,5,6-Tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA) hydrate is soluble in water at 16 mg/ml, but is insoluble in DMSO.
Legal Information
Sold in the USA under exclusive license from the University of California.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sowmya Venkataramani et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 30(46), 15664-15676 (2010-11-19)
Cells sensitive to the orientation of edges are ubiquitous in visual systems, and have been described in the vertebrate retina, yet the synaptic mechanisms that generate orientation selectivity in the retina are largely unknown. Here, we analyze the synaptic mechanisms
The first selective antagonist for a GABAC receptor.
Murata, Y., et al.
Bioorganic & Medicinal Chemistry Letters, 6, 2073-2076 (1996)
G A Johnston
Trends in pharmacological sciences, 17(9), 319-323 (1996-09-01)
The inhibitory neurotransmitter, GABA, activates a variety of receptors in all areas of the CNS. Two major subtypes of GABA receptors are well known: (1) GABAA receptors are ligand-gated Cl- channels that consist of a heteromeric mixture of protein subunits
D Ragozzino et al.
Molecular pharmacology, 50(4), 1024-1030 (1996-10-01)
In mammals, receptors for the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) are divided into three pharmacological classes, which are denoted GABAA, GABAB, and GABAC. GABAC receptors are defined by their insensitivity to the GABAA receptor antagonist bicuculline and the GABAB receptor
Cindy Karouta et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 35(9), e21846-e21846 (2021-08-19)
Myopia (short-sightedness), usually caused by excessive elongation of the eye during development, has reached epidemic proportions worldwide. In animal systems including the chicken model, several treatments have been shown to inhibit ocular elongation and experimental myopia. Although diverse in their
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service