SMB00311
Shogaol
≥90% (HPLC)
Synonym(s):
[6]-Shogaol, (6)-Shogaol, 1-(4-Hydroxy-3-methoxyphenyl)-4-decen-3-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H24O3
CAS Number:
Molecular Weight:
276.37
Beilstein:
2056098
MDL number:
UNSPSC Code:
12352205
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥90% (HPLC)
form
liquid
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
2-8°C
SMILES string
CCCCC\C=C\C(=O)CCc1ccc(O)c(OC)c1
InChI
1S/C17H24O3/c1-3-4-5-6-7-8-15(18)11-9-14-10-12-16(19)17(13-14)20-2/h7-8,10,12-13,19H,3-6,9,11H2,1-2H3/b8-7+
InChI key
OQWKEEOHDMUXEO-BQYQJAHWSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Shogaol or 6-shogaol, the pungent metabolite of dried ginger, is one of the main bioactive compounds extracted from the natural dietary rhizome, Zingiber officinale Roscoe (ginger). It is the dehydrated successor of 6-gingerol with an α,β-unsaturated ketone skeleton.
Application
Shogaol has been used to determine its safe dose to evaluate potential toxicity in higher concentrations in mice models. It has also been used to investigate its antioxidant effects on the modulation of TRPC5 (ITRPC5) and TRPA1 (ITRPA1) currents.
Biochem/physiol Actions
Shogaols exhibits higher potency, efficacy, and biological activities than gingerols. It is an excellent antioxidant agent, that protects human primary epidermal melanocytes against oxidative stress by activating nuclear factor E2-related factor (Nrf2)-antioxidant response. Shogaol also displays antimicrobial, antifungal, and anti-biofilm activities against Candida albicans, and Candida auris. It exerts anti-inflammatory properties and protects against abdomen irradiation (ABI)-induced intestinal side effects. 6-shogaol also inhibits lipopolysaccharide (LPS)-induced inflammation via peroxisome proliferator-activated receptor gamma (PPAR-γ) activation.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jin-Hyung Lee et al.
Frontiers in cellular and infection microbiology, 8, 299-299 (2018-09-14)
Candida albicans is an opportunistic pathogen and responsible for candidiasis. C. albicans readily forms biofilms on various biotic and abiotic surfaces, and these biofilms can cause local and systemic infections. C. albicans biofilms are more resistant than its free yeast
Lingli Yang et al.
International journal of molecular sciences, 21(10) (2020-05-21)
Skin is a major target of oxidative stress. Increasing evidence suggests that oxidative stress is the cause of melanocyte disappearance in vitiligo, which is an acquired pigmentary skin disorder characterized by patches of skin that have lost pigmentation. New herbal
H-R Kim et al.
Journal of applied microbiology, 130(4), 1142-1153 (2020-09-28)
This study aimed to assess the antifungal and anti-biofilm effects of 6-shogaol against Candida auris using in vitro phenotypic and genotypic analyses. Our results showed that 6-shogaol exhibited antifungal as well as anti-biofilm activity by inhibiting biofilm formation and eradicating
Assessment of Toxicological Effect of Shogaol in Albino Mice
Hassan S M and Hassan A H
Pakistan Veterinary Journal, 38(4), 377-383 (2018)
Young-Soo Kim et al.
International journal of molecular medicine, 38(6), 1905-1914 (2016-11-15)
Ginger extract is used as an analeptic in herbal medicine and has been reported to exert antioxidant effects. Transient receptor potential (TRP) canonical 5 (TRPC5), TRP cation channel, subfamily M, member 7 (TRPM7; melastatin 7), and TRP cation channel, subfamily A, member 1 (TRPA1; ankyrin 1) are non-selective cation channels that are
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[6]-Shogaol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/378/737/6e6bd2f9-0152-45e1-8006-344ca92937be/640/6e6bd2f9-0152-45e1-8006-344ca92937be.png)
![[6]-Gingerol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/259/444/6877889c-1cc0-47f5-b807-f847deadec1d/640/6877889c-1cc0-47f5-b807-f847deadec1d.png)
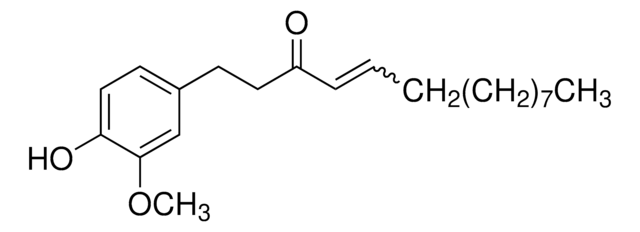
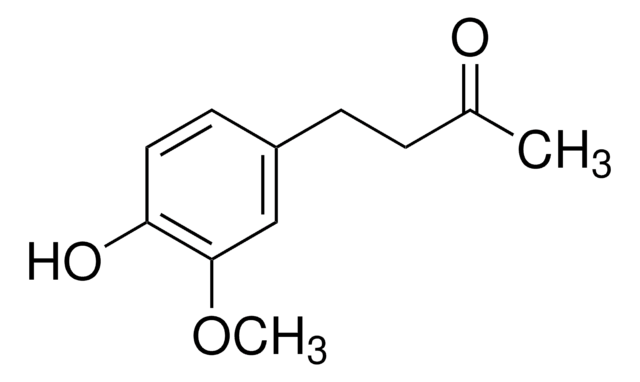
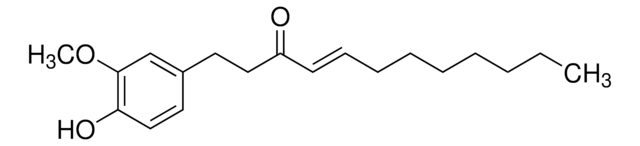
![[10]-Gingerol ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/224/210/b4f3e699-03b9-4112-89c1-a63f196344d0/640/b4f3e699-03b9-4112-89c1-a63f196344d0.png)