F6886
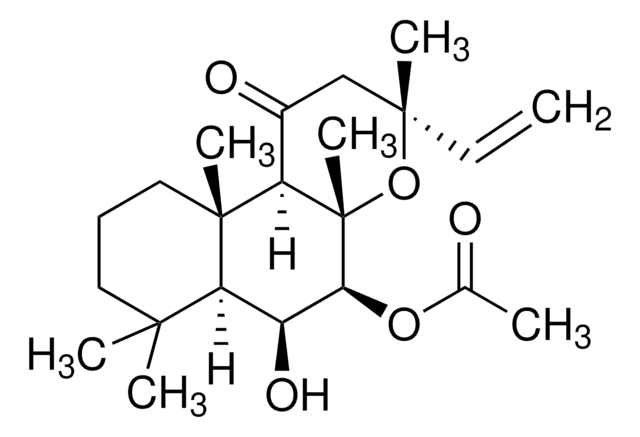
Forskolin
from Coleus forskohlii, ≥98% (HPLC), powder, positive ionotropic agent
Synonym(s):
7β-Acetoxy-8,13-epoxy-1α,6β,9α-trihydroxylabd-14-en-11-one, Coleonol, Colforsin
About This Item
Recommended Products
product name
Forskolin, from Coleus forskohlii, ≥98% (HPLC), powder
biological source
Coleus forskohlii
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white to off-white
solubility
ethanol: soluble 50 mg/mL
SMILES string
CC(=O)O[C@H]1[C@@H](O)[C@H]2C(C)(C)CC[C@H](O)[C@]2(C)[C@@]3(O)C(=O)C[C@@](C)(O[C@]13C)C=C
InChI
1S/C22H34O7/c1-8-19(5)11-14(25)22(27)20(6)13(24)9-10-18(3,4)16(20)15(26)17(28-12(2)23)21(22,7)29-19/h8,13,15-17,24,26-27H,1,9-11H2,2-7H3/t13-,15-,16-,17-,19-,20-,21+,22-/m0/s1
InChI key
OHCQJHSOBUTRHG-KGGHGJDLSA-N
Gene Information
human ... OPRK1(4986) , SLC2A10(81031) , TNF(7124)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- for inducing mRNA expression in primary hepatocytes.
- as a defatting drug in primary human hepatocytes.
- as a medium supplement to induce differentiation of adipose-derived stem cells (ASCs).
- in the activation of the cystic fibrosis transmembrane conductance regulator (CFTR) gene.
- to promote the differentiation of human induced pluripotent stem cells (hiPSCs) into endothelial cells.
- to stimulate cystic fibrosis transmembrane conductance regulator (CFTR)-mediated chloride ion secretion in differentiated airway epithelial cells derived from individuals with and without cystic fibrosis (CF).
Biochem/physiol Actions
Features and Benefits
Preparation Note
Storage and Stability
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Naive pluripotent stem cells are located within the epiblast of mature blastocysts. These primitive “ground-state” cells may be cultured in vitro using specialized media and small molecule inhibitors.
Protocols
A rapid in vitro assay for CFTR function, the forskolin-induced swelling protocol uses human colon organoids, which can be derived from cystic fibrosis patient tissue.
Related Content
Cyclic nucleotides, including cyclic AMP (cAMP), cyclic GMP (cGMP) and cyclic ADP-ribose, have been extensively studied as second messengers of intracellular events initiated by activation of GPCRs. cAMP modifies cell function in all eukaryotic cells, principally through the activation of cAMP-dependent protein kinase (PKA), but also through cAMP-gated ion channels and guanine nucleotide exchange factors directly activated by cAMP.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service