C9282
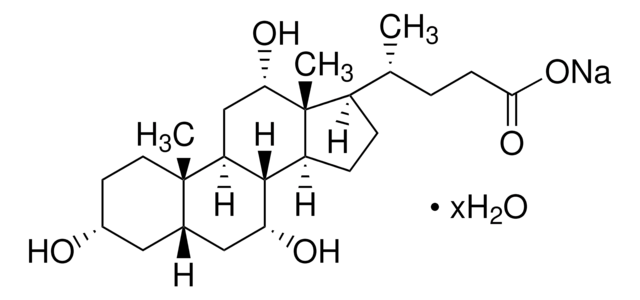
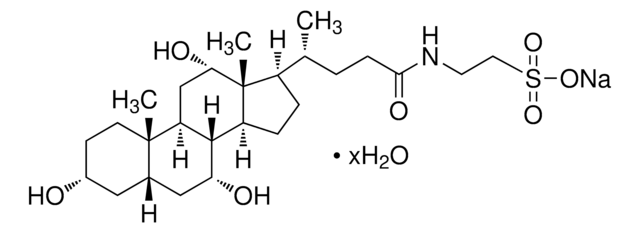
Sodium cholate hydrate
BioReagent, suitable for cell culture
Synonym(s):
3α,7α,12α-Trihydroxy-5β-cholan-24-oic acid sodium salt, Cholalic acid sodium salt
About This Item
Recommended Products
biological source
bovine bile
ovine bile
Quality Level
description
anionic
product line
BioReagent
Assay
≥99.0% (HPLC)
form
powder
mol wt
micellar avg mol wt 900-1300
aggregation number
2-3
technique(s)
cell culture | mammalian: suitable
CMC
9-15 mM (20-25°C)
solubility
water: 100 mg/mL, clear, colorless to light yellow
HLB
18
SMILES string
O.[Na+].C[C@H](CCC([O-])=O)C1CCC2C3[C@H](O)CC4C[C@H](O)CC[C@]4(C)C3C[C@H](O)[C@]12C
InChI
1S/C24H40O5.Na.H2O/c1-13(4-7-21(28)29)16-5-6-17-22-18(12-20(27)24(16,17)3)23(2)9-8-15(25)10-14(23)11-19(22)26;;/h13-20,22,25-27H,4-12H2,1-3H3,(H,28,29);;1H2/q;+1;/p-1/t13-,14+,15-,16-,17+,18+,19-,20+,22+,23+,24-;;/m1../s1
InChI key
MUVVIYFKOVLQHL-RCVKHMDESA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a bile acid to induce bile acid-responsive (BEAR) transcription system,
- as a component of Campylobacter defined broth (CDB) to test its effect on stress response in Campylobacter jejuni{52
- to test its sensitivity towards the bile acid sensor
Biochem/physiol Actions
recommended
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service