A6355
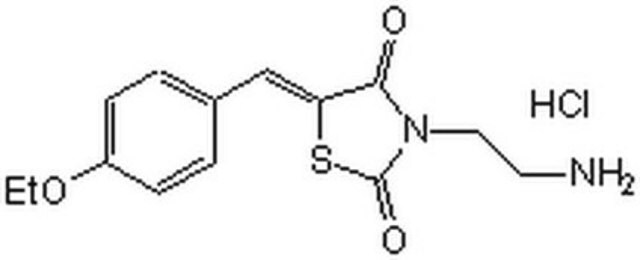
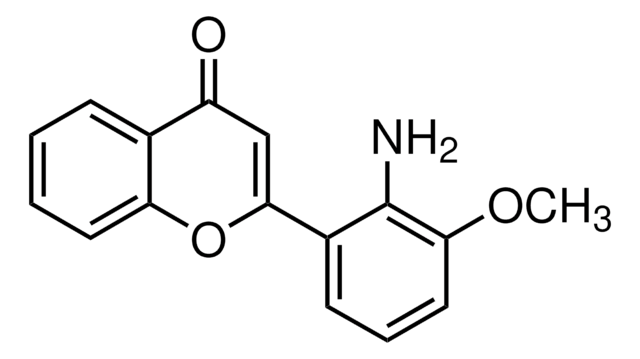
3-(2-Aminoethyl)-5-((4-ethoxyphenyl)methylene)-2,4-thiazolidinedione hydrochloride
powder, ≥98% (HPLC)
Synonym(s):
Erk Inhibitor
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H16N2O3S · HCl
CAS Number:
Molecular Weight:
328.81
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
storage condition
desiccated
under inert gas
color
off-white
solubility
DMSO: ≥4 mg/mL
storage temp.
2-8°C
SMILES string
Cl[H].CCOc1ccc(cc1)C=C2SC(=O)N(CCN)C2=O
InChI
1S/C14H16N2O3S.ClH/c1-2-19-11-5-3-10(4-6-11)9-12-13(17)16(8-7-15)14(18)20-12;/h3-6,9H,2,7-8,15H2,1H3;1H/b12-9+;
InChI key
PQVLWVGMXJPJLG-NBYYMMLRSA-N
Application
3-(2-Aminoethyl)-5-((4-ethoxyphenyl)methylene)-2,4-thiazolidinedione is an extracellular signal-regulated kinase (ERK) docking domain inhibitor. ERK inhibitors have been used to study traumatic brain injuries.
Biochem/physiol Actions
3-(2-Aminoethyl)-5-((4-ethoxyphenyl)methylene)-2,4-thiazolidinedione is extracellular signal-regulated kinase (ERK) docking domain inhibitor. Inhibits ERK binding rather then ERK activity at the ATP domain. IC50 = 25 μM in HeLa, A549, and SUM-159 tumor cells. Currently, no specific inhibitors of the ERK proteins exist. Preferentially binds to ERK2 with a Kd of ~5 μM and prevents its interaction with protein substrates. Shown to block ERK-mediated phosphorylation of ribosomal S6 kinase-1 (RSK-1) and ternary complex factor Elk-1, but exhibits little effect on ERK1/2 phosphorylation by MEK1/2.
Features and Benefits
This compound is featured on the MAPKs page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Dexmedetomidine is neuroprotective in an in vitro model for traumatic brain injury
Schoeler, M., et al.
BMC Neurology, doi: 10-doi: 10 (2012)
E Guerra et al.
Oncogene, 32(12), 1594-1600 (2012-05-09)
Our findings show that upregulation of a wild-type Trop-2 has a key controlling role in human cancer growth, and that tumour development is quantitatively driven by Trop-2 expression levels. However, little is known about the regulation of expression of the
Yiming Zhang et al.
The Journal of biological chemistry, 287(22), 18562-18572 (2012-04-12)
Although neuromedin U (NMU) has been implicated in analgesia, the detailed mechanisms still remain unclear. In this study, we identify a novel functional role of NMU type 1 receptor (NMUR1) in regulating the transient outward K(+) currents (I(A)) in small
Mikihito Kajiya et al.
Molecular pharmacology, 82(1), 115-124 (2012-04-19)
Muscarinic type 3 receptor (M3R) plays a pivotal role in the induction of glandular fluid secretions. Although M3R is often the target of autoantibodies in Sjögren's syndrome (SjS), chemical agonists for M3R are clinically used to stimulate saliva secretion in
Vikram Narayan et al.
The Journal of biological chemistry, 284(38), 25889-25899 (2009-06-09)
Our understanding of the post-translational processes involved in regulating the interferon regulatory factor-1 (IRF-1) tumor suppressor protein is limited. The introduction of mutations within the C-terminal Mf1 domain (amino acids 301-325) impacts on IRF-1-mediated gene repression and growth suppression as
Articles
We offers many products related to MAPKs for your research needs.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service