8.01641
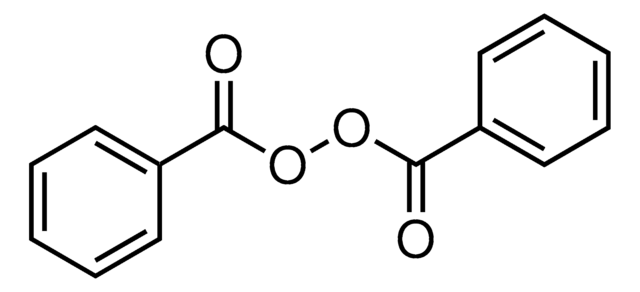
Benzoyl peroxide
(with 25% H2O) for synthesis
Synonym(s):
Benzoyl peroxide, Dibenzoyl peroxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H10O4
CAS Number:
Molecular Weight:
242.23
MDL number:
UNSPSC Code:
12352120
EC Index Number:
202-327-6
NACRES:
NA.22
Recommended Products
vapor pressure
<1 hPa ( 20 °C)
Quality Level
form
crystals
autoignition temp.
>380 °C
potency
7710 mg/kg LD50, oral (Rat)
reaction suitability
reagent type: oxidant
mp
100-105 °C (decomposition)
density
1.33 g/cm3 at 25 °C
bulk density
500‑600 kg/m3
functional group
peroxide
storage temp.
15-25°C
InChI
1S/C14H10O4/c15-13(11-7-3-1-4-8-11)17-18-14(16)12-9-5-2-6-10-12/h1-10H
InChI key
OMPJBNCRMGITSC-UHFFFAOYSA-N
General description
Benzoyl peroxide is an organic peroxide commonly used to initiate free radical polymerizations and to form copolymers through grafting reactions. It is also used as an oxidizer during chemical reactions.
Application
Benzoyl peroxide is used:
- In the direct N–O bond synthesis from 1,2-diamines.
- As an initiator in the synthesis of the cross-linked unsaturated copolymer by cross-linking methyl methacrylate with Poly (2-butene maleate) polyester.
Analysis Note
Assay (iodometric): 72,0 - 77,0 %(m)
Melting range (after drying): 102 - 105 °C
Identity (IR): conforms
Melting range (after drying): 102 - 105 °C
Identity (IR): conforms
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Org. Perox. C - Skin Sens. 1A
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 2
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Direct N--O bond formation via oxidation of amines with benzoyl peroxide
Banerjee A, et al.
Chemical Science, 10(71), 2124-2129 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service