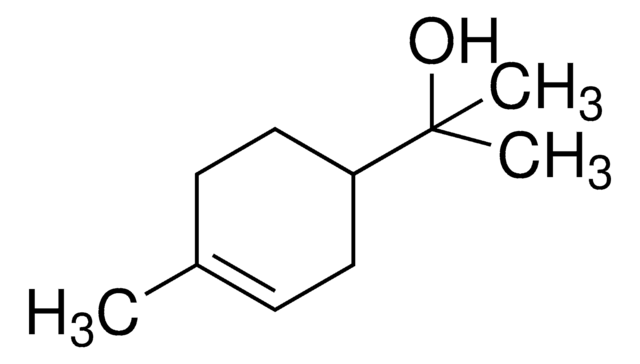
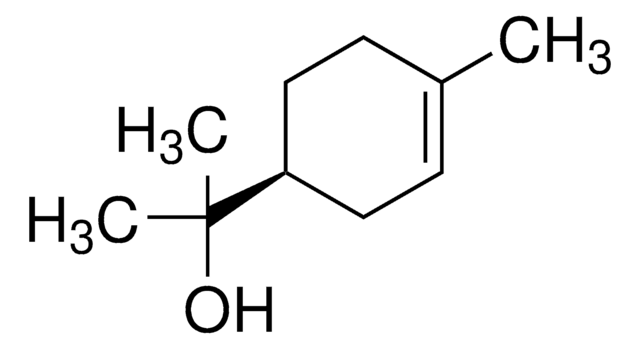
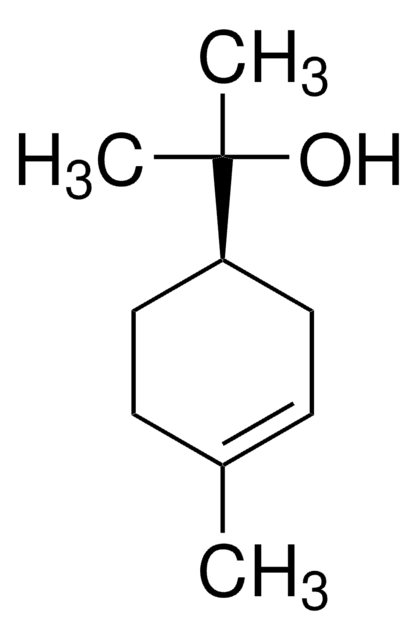
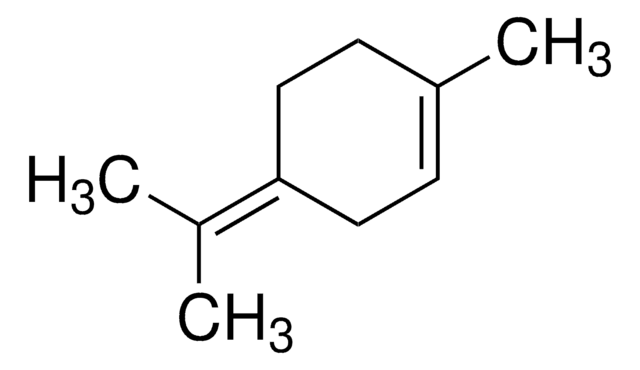
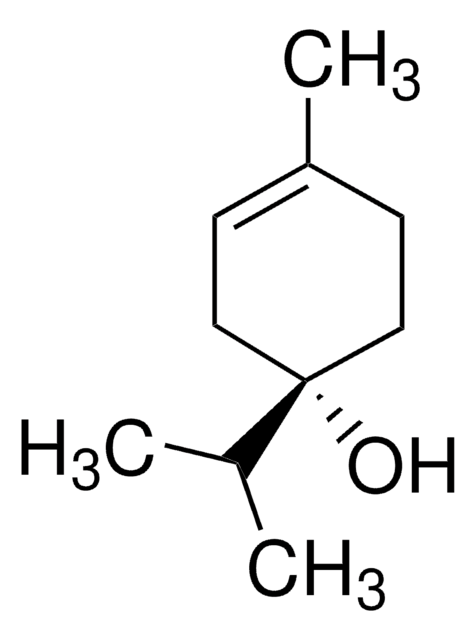
About This Item
Recommended Products
grade
analytical standard
Quality Level
Assay
≥90.0% (GC)
shelf life
limited shelf life, expiry date on the label
composition
γ-Terpineol, ≤10.0% GC (minor component)
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.480-1.486
n20/D 1.482 (lit.)
bp
217-218 °C (lit.)
mp
31-35 °C (lit.)
density
0.93 g/mL at 25 °C (lit.)
application(s)
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
format
neat
SMILES string
CC1=CC[C@H](CC1)C(C)(C)O
InChI
1S/C10H18O/c1-8-4-6-9(7-5-8)10(2,3)11/h4,9,11H,5-7H2,1-3H3
InChI key
WUOACPNHFRMFPN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
195.8 °F - closed cup
Flash Point(C)
91 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
-Cymene; Linalool; Menthol; α-Terpineol; Menthyl acetate
Protocols
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
-α-Bergamotene; β-Bisabolene; α-Terpineol; Neryl acetate; Geranyl acetate; Neral; Geranial
GC Analysis of Sweet Orange Essential Oil on SLB®-5ms (10 m x 0.10 mm I.D., 0.10 μm), Fast GC Analysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service