114669
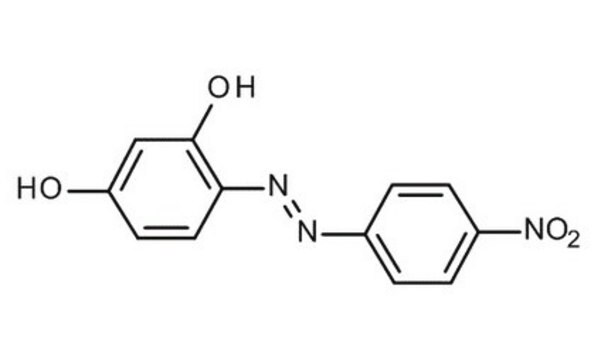
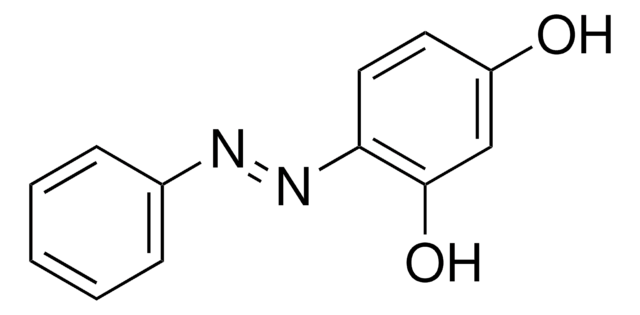
4-(4-Nitrophenylazo)resorcinol
Dye content 90 %
Synonym(s):
Azoviolet, Magneson I
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
O2NC6H4N=NC6H3-1,3-(OH)2
CAS Number:
Molecular Weight:
259.22
Beilstein:
674709
EC Number:
MDL number:
UNSPSC Code:
12171500
PubChem Substance ID:
NACRES:
NA.47
Recommended Products
form
powder
Quality Level
composition
Dye content, 90%
mp
195-200 °C (dec.) (lit.)
λmax
432 nm
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
Oc1ccc(\N=N\c2ccc(cc2)[N+]([O-])=O)c(O)c1
InChI
1S/C12H9N3O4/c16-10-5-6-11(12(17)7-10)14-13-8-1-3-9(4-2-8)15(18)19/h1-7,16-17H/b14-13+
InChI key
NGPGYVQZGRJHFJ-BUHFOSPRSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Biochem/physiol Actions
4-(4-Nitrophenylazo) resorcinol, also known as azo violet or magneson I, is a red colored powder. It functions as a spot test reagent and forms blue lake on reaction with Mg(OH)2 in alkaline solution. It is mainly used for determining the presence of magnesium in the solution.
4-(4-Nitrophenylazo)resorcinol is a pH indicator.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kuang Lu Cheng, Keihei Ueno, Toshiaki Imamura
CRC Handbook of Organic Analytical Reagents (1992)
Cheng K L
CRC Handbook of Organic Analytical Reagents (1992)
R. Gopalan
Inorganic Chemistry for Undergraduates (2009)
National Research Council (U.S.). Food Protection Committee
Food Chemicals Codex (1967)
Highly sensitive determination method for total carbonate in water samples by flow injection analysis coupled with gas-diffusion separation.
Oshima M, et al.
Analytical Sciences, 17, 1285-1290 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service