07551
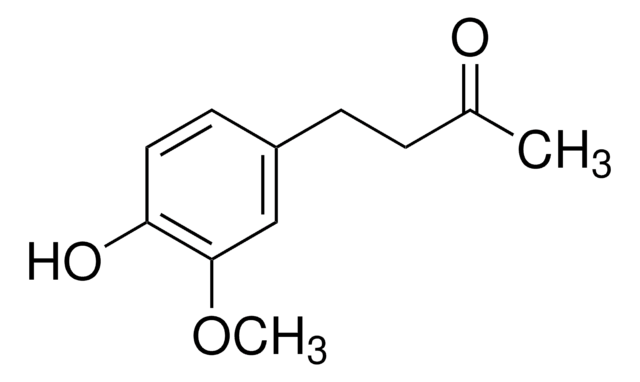
Vanillylidenacetone
≥98.5%
Synonym(s):
4-(4-Hydroxy-3-methoxyphenyl)-3-buten-2-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H12O3
CAS Number:
Molecular Weight:
192.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.5%
color
yellow
mp
125-130 °C
functional group
ketone
SMILES string
COc1cc(\C=C\C(C)=O)ccc1O
InChI
1S/C11H12O3/c1-8(12)3-4-9-5-6-10(13)11(7-9)14-2/h3-7,13H,1-2H3/b4-3+
InChI key
AFWKBSMFXWNGRE-ONEGZZNKSA-N
Gene Information
human ... APP(351)
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pharmacological actions and acute toxicity of methyl- and phenyl-3-methoxy-4-hydroxy styryl ketones.
G B Singh et al.
Arzneimittel-Forschung, 37(6), 708-712 (1987-06-01)
Some pharmacological actions and acute toxicity effects of methyl- and phenyl-3-methoxy-4-hydroxy styryl ketones have been described in experimental animals. The compounds antagonised the contractions evoked by a variety of agonists on several smooth muscle preparations in vitro. They produced inhibitory
G B Singh et al.
Arzneimittel-Forschung, 37(4), 435-440 (1987-04-01)
Methyl- and phenyl-3-methoxy-4-hydroxy styryl ketones (MHSK and PHSK, resp.) upon oral administration displayed marked antiinflammatory activity in a variety of acute tests viz. carrageenan, histamine, 5-hydroxytryptamine, dextran, bradykinin and prostaglandin (PG) induced oedema in rats and carrageenan evoked swelling in
Shingo Yogosawa et al.
Journal of natural products, 75(12), 2088-2093 (2012-12-19)
Dehydrozingerone (1) is a pungent constituent present in the rhizomes of ginger (Zingiber officinale) and belongs structurally to the vanillyl ketone class. It is a representative of half the chemical structure of curcumin (2), which is an antioxidative yellow pigment
Vipan Kumar Parihar et al.
Chemico-biological interactions, 170(1), 49-58 (2007-09-04)
Dehydrozingerone (DZ) was explored for in vitro-in vivo antioxidant potential and in vivo radioprotective activity against whole body gamma irradiation in Swiss albino mice. DZ scavenged the ABTS (2, 2'-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) and DPPH (1, 1-dipehnyl-2-picrylhydrazyl) free radicals at room
D V Rajakumar et al.
Molecular and cellular biochemistry, 140(1), 73-79 (1994-11-09)
The present study investigates the inhibition of lipid peroxidation by dehydrozingerone and curcumin in rat brain homogenates. Both the test compounds inhibited the formation of conjugated dienes and spontaneous lipid peroxidation. These compounds also inhibited lipid peroxidation induced by ferrous
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service