COHISC-RO
Roche
cOmplete™ His-Tag Purification Column
Prepacked, ready-to-use chromatography column
About This Item
Recommended Products
packaging
pkg of 5 x 1 mL (columns (06781543001))
pkg of 5 mL (column (06781535001))
manufacturer/tradename
Roche
parameter
1,420 cm/hr max. flow rate
capacity
≥40 mg/mL, resin binding capacity
storage temp.
2-8°C
Related Categories
General description
Features and Benefits
cOmplete His-Tag Purification Columns are fully compatible with standard purification systems such as ÄKTA Systems (Cytiva).
- Use the buffer conditions best suited to your protein
- Repeatedly obtain highly pure protein
- Protect your protein from toxic nickel
- Work in a safe and eco-friendly environment
Packaging
- 06781535001 (5 mL column; 1 column containing 5 ml of cOmplete His-Tag Purification Resin)
- 06781543001 (5 x 1 mL columns; 5 columns containing 1 ml of cOmplete His-Tag Purification Resin)
Compatibility
Compatibility during chromatography: The resin is compatible with 10mM EDTA, 10mM DTT during the purification (1 hour incubation), 6M guanidinium-HCl, 8M urea, pH 2.0 to pH 14.0.
Compatibility during cleaning: 4% SDS Form cOmplete His-Tag Purification Resin filled in columns, pre-charged with Ni2+ stored in 20% ethanol.
Other Notes
1 ml column (06 781 543 001): 0.5 to 2.0 ml/minute
The volumetric flow rate is a function of the column′s cross section.
Recommended imidazole concentration for load/wash: Nonspecific binding of proteins without a His-tag is low.
Use up to 5 mM imidazole in load and/or wash buffers.
If establishing a new assay for purification of His-tag proteins with cOmplete His-Tag Purification Columns do not use imidazole. If, e.g., the purity of the His-tag protein needs to be improved following this first step, use imidazole in a final concentration of up to 5mM in a second step.
Recommended imidazole concentration for elution: Up to 500mM
Please note:
In contrast to other available resins, bound His-tagged protein typically elutes from cOmplete His-Tag Purification Columns with a lower imidazole concentration, e.g., 25 to 45mM.
Binding capacity
The binding capacity of the resin to various types of proteins may vary according to the protein characteristics such as the size of the protein.
cOmplete His-Tag Purification Columns bind with a high specificity to the polyhistidine-tagged protein. As a consequence, the binding kinetics may appear to be different when compared to conventional metal chelate matrices. Full capacity of cOmplete His-Tag Purification Columns can be achieved by allowing more time for the protein to bind to the resin by lowering the flow rate during the chromatography purification procedure.
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
93.2 °F
Flash Point(C)
34 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Get maximum protection after protein isolation by using protease and phosphatase inhibitor cocktail tablets from Roche.
Protocols
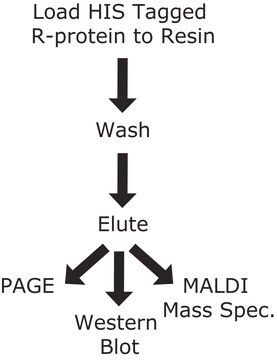
Purification Protocols: cOmplete His-Tag Purification Columns are compatible with automated chromatography systems such as ÄKTAexplorer.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service