1.05454
GranuCult® prime BRILA (Brilliant-green bile Lactose) broth
according to ISO 4831:2006, ISO 4832:2006, FDA-BAM, for Escherichia coli, for coliforms, suitable for microbiology
Synonym(s):
BGB broth, BGBLB, Brilliant Green Bile broth, Brilliant-green bile lactose broth, Brilliant-green bile lactose broth, Brillant-green bile lactose broth
About This Item
Recommended Products
Agency
SMWW Part 9221
APHA
FDA-BAM
GB 4789.3:2016
ISO 4832:2006
according to ISO 4831:2006
Quality Level
sterility
non-sterile
form
granular
packaging
pkg of 5 kg
pkg of 500 g
manufacturer/tradename
GranuCult® prime
storage condition
dry media (Keep tightly closed)
technique(s)
microbiological culture: suitable
color
green
pH
7.2 (30 °C, 40 g/L in H2O, after autoclaving)
solubility
40 g/L
bulk density
560 kg/m3
application(s)
food and beverages
microbiology
storage temp.
15-25°C
suitability
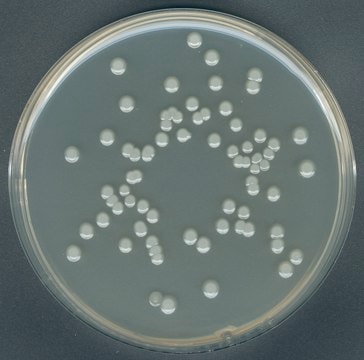
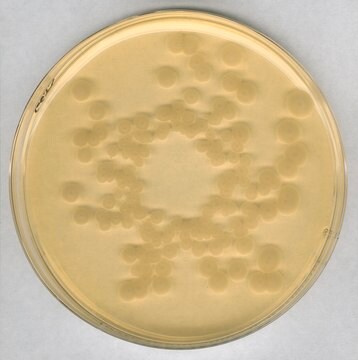
Escherichia coli
coliforms
General description
Application
Features and Benefits
- GranuCult® offers superior granulated culture media
- Safe and sustainable due to reduced risks associated with fine dust and toxic substance inhalation, resulting in a safer work environment
- Excellent wettability, solubility, and free-flowing properties
- Convenient, with minimal component separation and clumping, even under warm or humid conditions
- High batch-to-batch reproducibility
- Prolonged shelf life of up to five years
- High number of test strains exceeding all regulatory demands
- Granulation technology allows many supplements to be included, with no need to add these separately
Footnote
The designations basic, plus, or prime are added to indicate the quality control level, from basic quality control to standard QC plus to prime for full regulatory compliance.
Legal Information
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Microbial culture media is available in both powdered and granulated forms. This article compares the characteristics of each culture media format with regards to safety, handling and convenience.
See how two water sources compare when preparing and performance testing microbiological culture media acc. to EN ISO 11133: centrally purified water vs. water from a Milli-Q® IX system were assessed. Tests included media specific for Listeria and Salmonella pathogens.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service