W318809
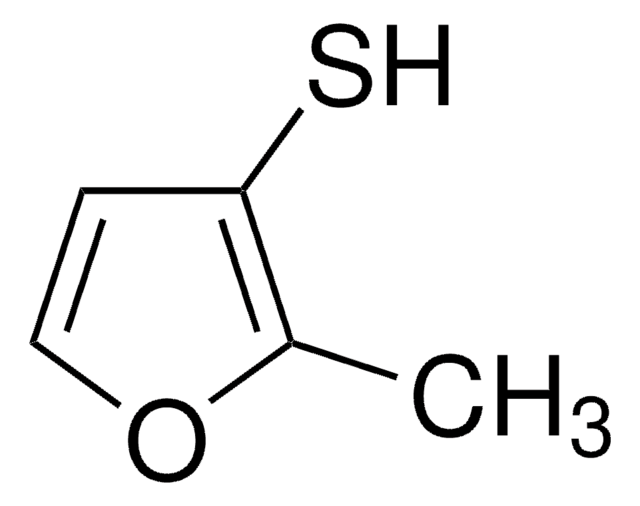
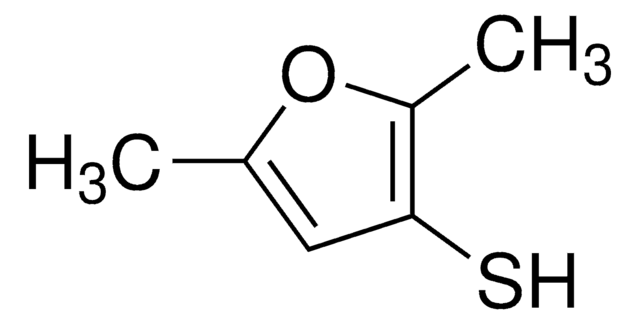
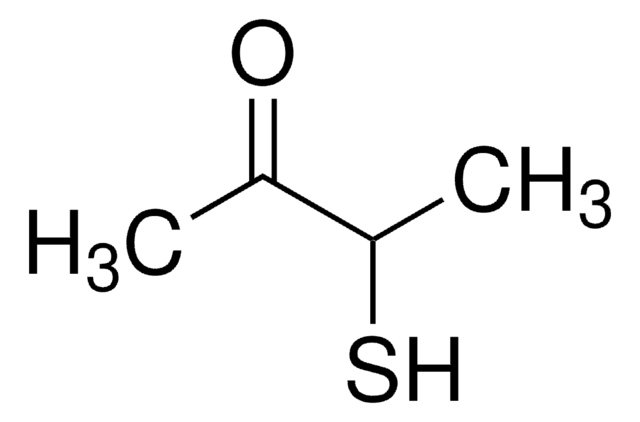
2-Methyl-3-furanthiol
≥95%, FG
Synonym(s):
2-methyl-3-furyl mercaptan, Fish thiol, Oxy cyclothione-030
About This Item
Fragrance grade
Halal
Kosher
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
vapor density
>1 (vs air)
Assay
≥95%
refractive index
n20/D 1.518 (lit.)
bp
57-60 °C/44 mmHg (lit.)
density
1.145 g/mL at 25 °C
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
fishy; meaty; metallic; sulfurous
SMILES string
Cc1occc1S
InChI
1S/C5H6OS/c1-4-5(7)2-3-6-4/h2-3,7H,1H3
InChI key
RUYNUXHHUVUINQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Elucidating salt-reduction mechanisms of aroma-active compounds from yeast extracts through sensomics approaches and electroencephalography.: This innovative study leverages both sensory science and neurotechnology to uncover how 2-methyl-3-furanthiol and other key compounds in yeast extracts can enhance flavor profiles while reducing sodium content in foods, aligning with health trends and consumer demands for lower-sodium options (Shan et al., 2024).
- Widely Targeted Metabolomics and Network Pharmacology Reveal the Nutritional Potential of Yellowhorn (Xanthoceras sorbifolium Bunge) Leaves and Flowers.: This study demonstrates the use of targeted metabolomics to profile bioactive compounds, including 2-methyl-3-furanthiol, in Yellowhorn plant parts, which are emerging as a nutritional powerhouse with potential health benefits (Sha et al., 2024).
- Metabolomics reveals factors affecting the radical reaction of sulfides during thermal processing for meaty aroma.: This research identifies how 2-methyl-3-furanthiol and other sulfides interact during cooking to create rich, meaty flavors, providing valuable insights for the culinary industry on optimizing flavor profiles in cooked meats (Zhang et al., 2024).
Biochem/physiol Actions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
98.6 °F - closed cup
Flash Point(C)
37 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service