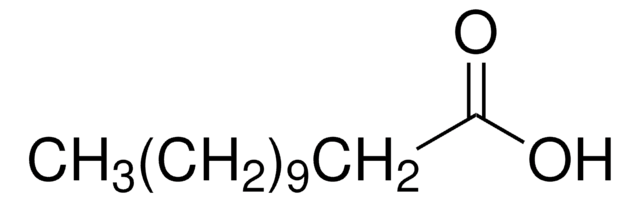W276413
Myristic acid
natural, ≥98.5%, FG
Synonym(s):
1-Tridecanecarboxylic acid, C14:0, NSC 5028, Tetradecanoic acid
About This Item
Fragrance grade
Halal
Kosher
natural
Recommended Products
grade
FG
Fragrance grade
Halal
Kosher
natural
Quality Level
Agency
follows IFRA guidelines
meets purity specifications of JECFA
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
Assay
≥98.5%
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
bp
250 °C/100 mmHg (lit.)
mp
52-54 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
greener alternative category
Organoleptic
oily; waxy; soapy
SMILES string
CCCCCCCCCCCCCC(O)=O
InChI
1S/C14H28O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14(15)16/h2-13H2,1H3,(H,15,16)
InChI key
TUNFSRHWOTWDNC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Experiments and Molecular Simulations to Study the Effect of Surface-Active Compounds in Mixtures of Model Oils on CO2 Corrosion during Intermittent Oil-Water Wetting.: Investigates how myristic acid, as a surface-active compound, influences CO2 corrosion mechanisms in oil-water systems, offering insights into corrosion prevention strategies in the oil industry (Norooziasl et al., 2024).
Disclaimer
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service