P49805
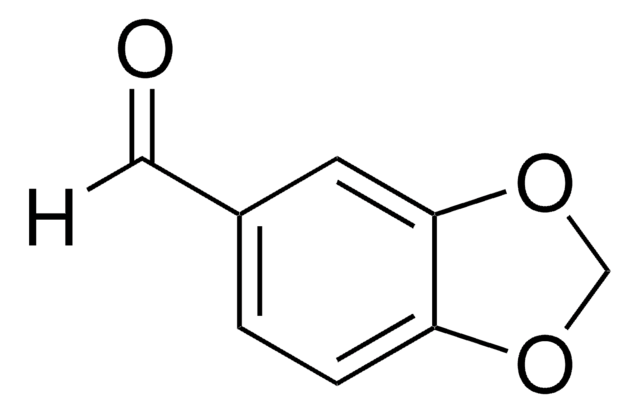
Piperonylic acid
99%
Synonym(s):
1,3-Benzodioxole-5-carboxylic acid, 3,4-(Methylenedioxy)benzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H6O4
CAS Number:
Molecular Weight:
166.13
Beilstein:
150206
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
229-231 °C (lit.)
SMILES string
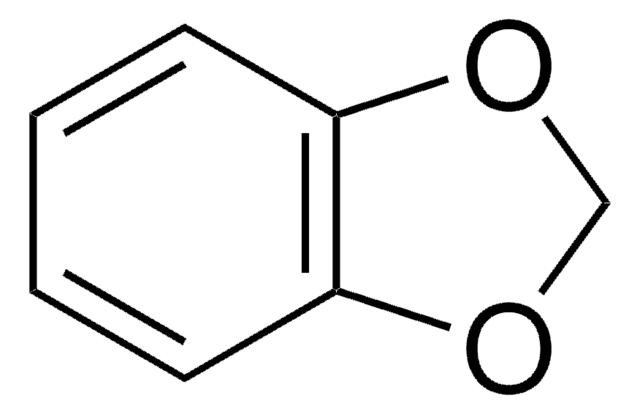
OC(=O)c1ccc2OCOc2c1
InChI
1S/C8H6O4/c9-8(10)5-1-2-6-7(3-5)12-4-11-6/h1-3H,4H2,(H,9,10)
InChI key
VDVJGIYXDVPQLP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R Ranga Rao et al.
Bioorganic & medicinal chemistry, 17(14), 5170-5175 (2009-06-12)
A bioassay-guided fractionation and chemical examination of antihyperglycemic root extract of Derris indica resulted in isolation and characterization of two new furanoflavanoids (1, 2) along with thirteen known compounds (3-15). Their structures were determined on the basis of extensive spectroscopic
Debabrata Sircar et al.
Journal of plant physiology, 166(13), 1370-1380 (2009-04-04)
Biosynthesis of hydroxybenzoates even at enzymatic level is poorly understood. In this report, effect of feeding of putative biosynthetic precursors and pathway-specific enzyme inhibitors of early phenylpropanoid pathway on p-hydroxybenzoic acid accumulation in chitosan-elicited hairy roots of Daucus carota was
Eyal Shimoni et al.
Journal of biotechnology, 105(1-2), 61-70 (2003-09-27)
Propenylbenzenes are often used as starting materials in the chemical synthesis of aroma compounds and fine chemicals. In the present study, we demonstrate the ability of an Arthrobacter sp. to transform various structures of propenylbenzenes derived from essential oils to
Gisele Adriana Bubna et al.
Journal of plant physiology, 168(14), 1627-1633 (2011-04-15)
The allelopathic effect of caffeic acid was tested on root growth, phenylalanine ammonia-lyase (PAL) and peroxidase (POD) activities, hydrogen peroxide (H(2)O(2)) accumulation, lignin content and monomeric composition of soybean (Glycine max) roots. We found that exogenously applied caffeic acid inhibited
J Chong et al.
Plant physiology, 125(1), 318-328 (2001-01-12)
Salicylic acid (SA) is a key endogenous component of local and systemic disease resistance in plants. In this study, we investigated the role of benzoic acid (BA) as precursor of SA biosynthesis in tobacco (Nicotiana tabacum cv Samsun NN) plants
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service