All Photos(2)
About This Item
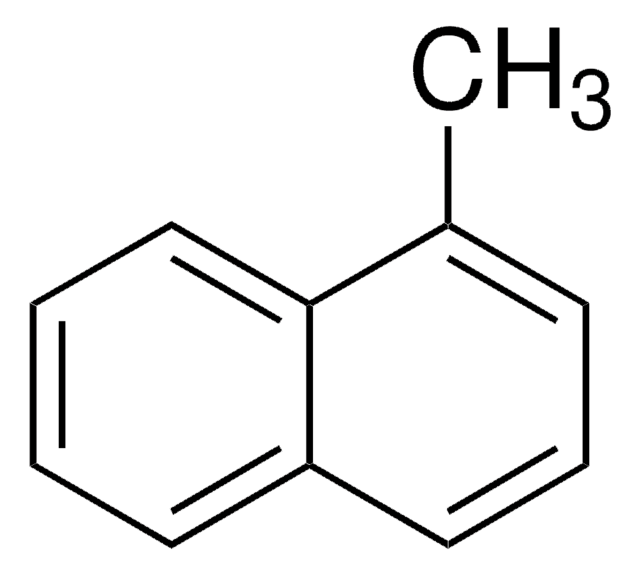
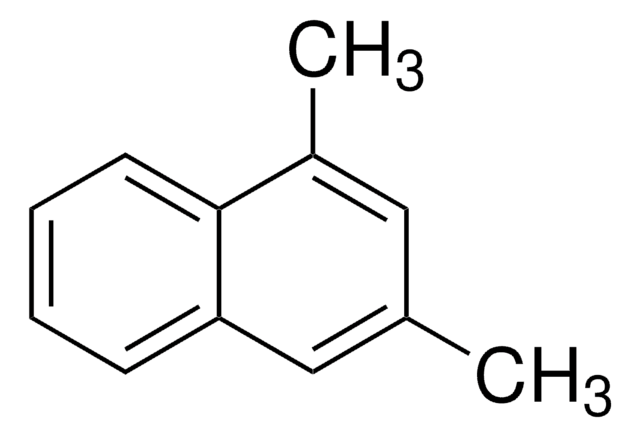
Linear Formula:
C10H7CH3
CAS Number:
Molecular Weight:
142.20
Beilstein:
506793
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
autoignition temp.
984 °F
refractive index
n20/D 1.615 (lit.)
bp
240-243 °C (lit.)
mp
−22 °C (lit.)
density
1.001 g/mL at 25 °C (lit.)
SMILES string
Cc1cccc2ccccc12
InChI
1S/C11H10/c1-9-5-4-7-10-6-2-3-8-11(9)10/h2-8H,1H3
InChI key
QPUYECUOLPXSFR-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
Application
1-Methylnaphthalene can be used in:
- The synthesis of various polycyclic aromatic hydrocarbons (PAHs), including naphtho[a]carbazoles and naphthopyrones.
- The preparation of 1-methylnaphthalene based tocainide analog as skeletal muscle voltage-gated sodium channel blocker.
- The synthesis of aminobenzoyl-2-hydroxy-1-naphthyl hydrazine, a potential HIV reverse-transcriptase-DNA-polymerase inhibitor (RTI).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Asp. Tox. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A versatile synthesis of annulated carbazole analogs involving a domino reaction of bromomethylindoles with arenes/heteroarenes.
Dhayalan V, et al.
European Journal of Organic Chemistry, 2009(4), 531-546 (2009)
From 1-Methylnaphthalene to Aminobenzoyl-2-Hydroxy-1-naphthyl hydrazone.
Genov K and Nikolova D
Journal of the University of Chemical Technology and Metallurgy, 44(3), 281-285 (2009)
N-Aryl-2, 6-dimethylbenzamides, a new generation of tocainide analogues as blockers of skeletal muscle voltage-gated sodium channels.
Muraglia M, et al.
Journal of Medicinal Chemistry, 57(6), 2589-2600 (2014)
A concise synthesis of novel naphtho [a] carbazoles and benzo [c] carbazoles.
Pathak R, et al.
Tetrahedron, 62(12), 2820-2830 (2006)
Pigments of marine animals. XII. The synthesis of certain substituted naphthopyrones related to crinoid pigments.
Rideout JA, et al.
Australian Journal of Chemistry, 29(5), 1087-1098 (1976)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service