All Photos(1)
About This Item
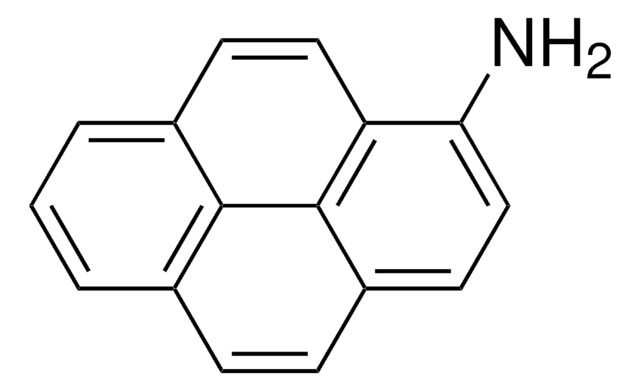
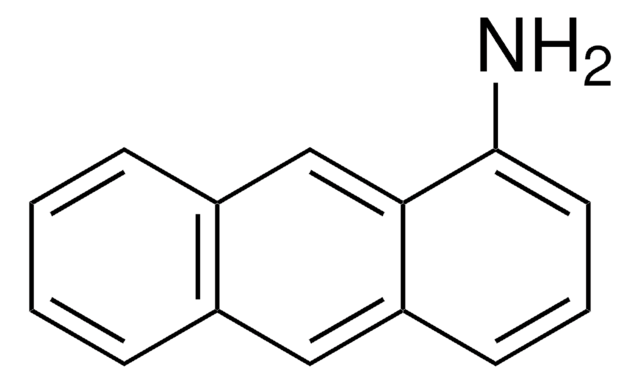
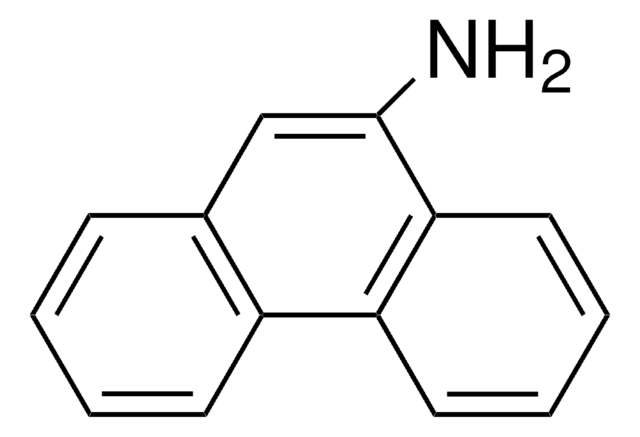
Empirical Formula (Hill Notation):
C18H13N
CAS Number:
Molecular Weight:
243.30
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
solid
Assay:
95%
Recommended Products
Quality Level
Assay
95%
form
solid
mp
209-211 °C (lit.)
SMILES string
Nc1cc2c3ccccc3ccc2c4ccccc14
InChI
1S/C18H13N/c19-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H,19H2
InChI key
KIVUHCNVDWYUNP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Produces tumors in mice.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B G Lake et al.
Toxicology and applied pharmacology, 138(2), 231-241 (1996-06-01)
Precision-cut liver slices were prepared from male Sprague-Dawley rats (pretreated with or without Aroclor 1254), male Dunkin-Hartley guinea pigs, male cynomolgus monkeys, and humans. Liver slices were cultured for 24 hr using a dynamic organ culture system in medium containing
H Yamazaki et al.
Carcinogenesis, 15(3), 465-470 (1994-03-01)
In order to address the hypothesis that 6-aminochrysene (6-AC) is converted to genotoxic products by cytochrome P450 enzymes via two activation pathways (N-hydroxylation and epoxidation), the activation of 6-AC and trans-1,2-dihydro-1,2-dihydroxy-6-aminochrysene (6-AC-diol) to genotoxic metabolites was examined in rat and
K B Delclos et al.
Carcinogenesis, 8(11), 1703-1709 (1987-11-01)
Since 6-nitrochrysene and 6-aminochrysene have shown activity in carcinogenicity bioassays, we have begun an investigation of their metabolic activation pathways and the nature of the carcinogen-DNA adducts that may be formed. N-Hydroxy-6-aminochrysene (N-hydroxy-AC), a candidate proximate or ultimate carcinogen and
K B Delclos et al.
IARC scientific publications, (124)(124), 79-86 (1993-01-01)
Carcinogenic arylamines and nitroaromatic hydrocarbons are chemicals that present occupational health hazards and share pathways of metabolic activation. The 32P-postlabelled DNA adducts formed in Chinese hamster ovary (CHO) cells treated with metabolites from two pathways that are common to the
M Mimura et al.
Drug metabolism and disposition: the biological fate of chemicals, 21(6), 1048-1056 (1993-11-01)
A cytochrome P-450 (P-450) enzyme of the CYP2B subfamily was partially purified from human liver microsomes and characterized with respect to immunochemical properties, N-terminal amino acid sequence, and catalytic activities toward typical P-450 substrates. P-450 enzymes were monitored in chromatographic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service