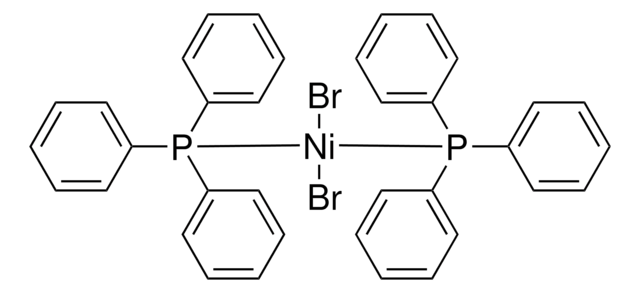
913278
[1,2-Bis(diphenylphosphino)ethane]dibromonickel(II)
≥95%
Synonym(s):
dppeNiBr2
About This Item
Recommended Products
Assay
≥95%
form
powder
reaction suitability
reagent type: catalyst
reaction type: Cross Couplings
mp
322-326 °C
SMILES string
[Ni+2].P(CCP(c4ccccc4)c3ccccc3)(c2ccccc2)c1ccccc1.[Br-].[Br-]
InChI
1S/C26H24P2.2BrH.Ni/c1-5-13-23(14-6-1)27(24-15-7-2-8-16-24)21-22-28(25-17-9-3-10-18-25)26-19-11-4-12-20-26;;;/h1-20H,21-22H2;2*1H;/q;;;+2/p-2
InChI key
AEGMSHMBRVOLMV-UHFFFAOYSA-L
Application
- kumada catalyst-transfer polycondensation
- oxidation of carboranyl phosphine ligands
- synthesis of nickel-iron dithiolato hydrides
- hydroformylation of alcohols
- Ullmann reactions - homocoupling reactions
- intramolecular aerobic oxidative amination of alkenes
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A Inhalation - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1 - STOT RE 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,2-Bis(diphenylphosphino)ethane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/707/956/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf/640/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf.png)
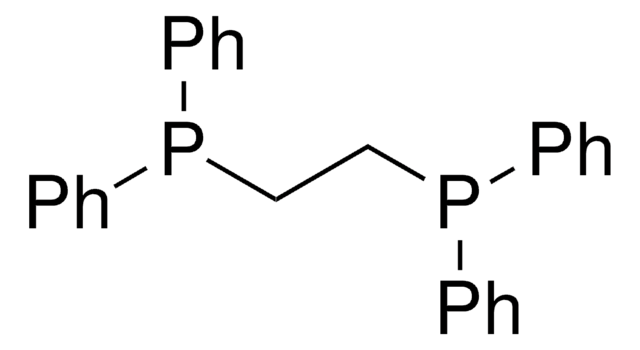
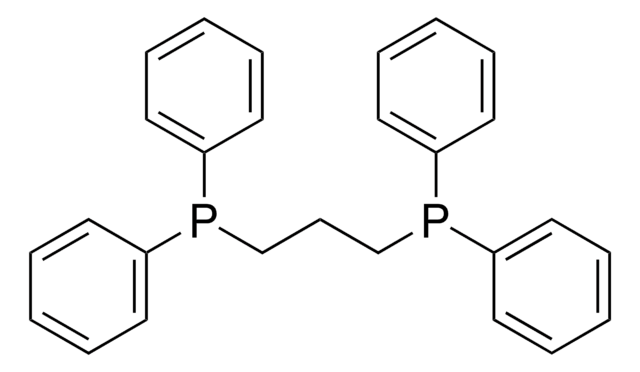
![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)
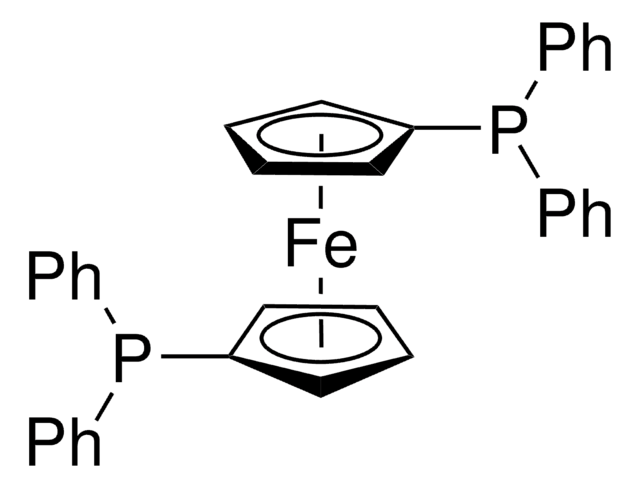
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloronickel(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/274/566/a60d6584-163a-4c41-a738-60f8e4d524fa/640/a60d6584-163a-4c41-a738-60f8e4d524fa.png)