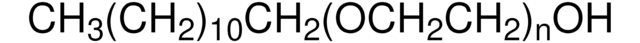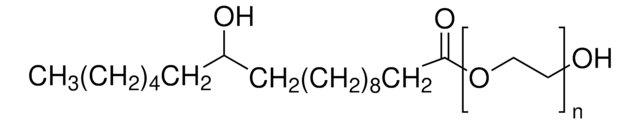698717
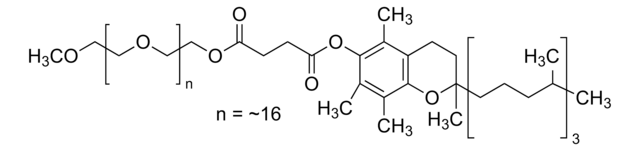
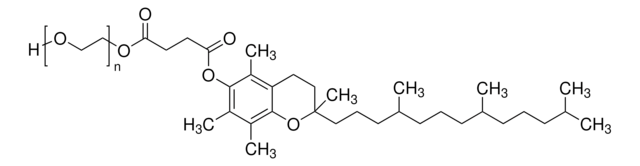
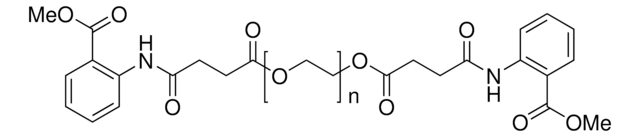
Polyoxyethanyl-α-tocopheryl sebacate
15 wt. % in H2O
Synonym(s):
PTS
About This Item
Recommended Products
form
liquid
mol wt
~1,200
reaction suitability
reaction type: C-C Bond Formation
greener alternative product characteristics
Waste Prevention
Designing Safer Chemicals
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
concentration
15 wt. % in H2O
greener alternative category
General description
Application
Uses:
- Provides an aqueous micellar environment for transition metal-catalyzed Heck coupling, cross metathesis and ring closing metathesis reactions
- Surfactant for catalytic asymmetric Baeyer-Villiger oxidation in water using PtII catalysts and hydrogen peroxide
- Orally active CoQ10 carrier for neuroprotection after stroke or cardiac arrest
Legal Information
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Micellular catalysis has provided the ability to carry out several commonly used transformations used in the synthetic community to be carried out in water.
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
Related Content
Prof. Bruce Lipshutz and co-workers have developed designer surfactants to allow several classes of transformations (e.g. Suzuki-Miyaura, Olefin Metathesis, 1,4-Addition to Enones, etc.) to be performed in water.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service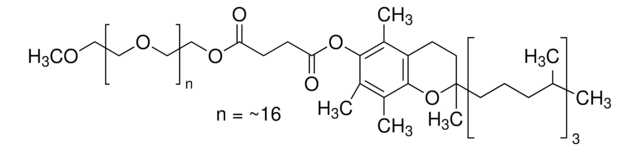


![[1,1′-Bis(di-tert-butylphosphino)ferrocene]dichloropalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/192/459/02d1239c-1119-49d9-b392-a04d8f53855c/640/02d1239c-1119-49d9-b392-a04d8f53855c.png)