638455
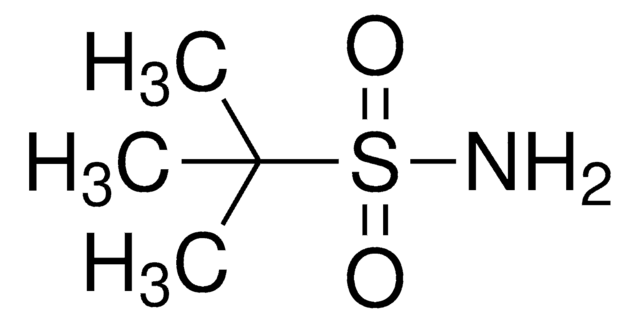
Trifluoromethanesulfonamide
95%
Synonym(s):
1,1,1-Trifluoromethanesulfonamide, Trifluoromethylsulfonamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3SO2NH2
CAS Number:
Molecular Weight:
149.09
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Application
Trifluoromethanesulfonamide can undergo reaction with paraformaldehyde either in sulfuric acid to give the corresponding open chain and cyclic condensation products or in ethyl acetate to give the corresponding oxy-methylated products.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C J Lynch et al.
The Biochemical journal, 310 ( Pt 1), 197-202 (1995-08-15)
The role of carbonic anhydrase in de novo lipid synthesis was examined by measuring [1-14C]acetate incorporation into total lipids, fatty acids and non-saponifiable lipids in freshly isolated rat hepatocytes. Two carbonic anhydrase inhibitors, trifluoromethylsulphonamide (TFMS) and ethoxozolamide (ETZ) decreased incorporation
"Oxymethylation of trifluoromethanesulfonamide with paraformaldehyde in ethyl acetate"
Meshcheryakov.I.V, et al.
Russ. J. Org. Chem., 44(02), 311-316 (2008)
Marine Soulié et al.
Physical chemistry chemical physics : PCCP, 18(43), 29999-30008 (2016-11-03)
The arrangement of an ionic fluorophore in the crystalline state was regulated by the presence of various counter-ions and the effect on spectroscopic and self-association properties was studied. To do so, nine salts of berberine (i.e. a fluorescent natural alkaloid)
"Cascade transformations of trifluoromethanesulfonamide in reaction with formaldehyde"
Meshcheryakov.I.V, et al.
Russ. J. Org. Chem., 41(09), 1381-1386 (2005)
Lina Baranauskienė et al.
BMC biophysics, 5, 12-12 (2012-06-09)
Human carbonic anhydrases (CAs) play crucial role in various physiological processes including carbon dioxide and hydrocarbon transport, acid homeostasis, biosynthetic reactions, and various pathological processes, especially tumor progression. Therefore, CAs are interesting targets for pharmaceutical research. The structure-activity relationships (SAR)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service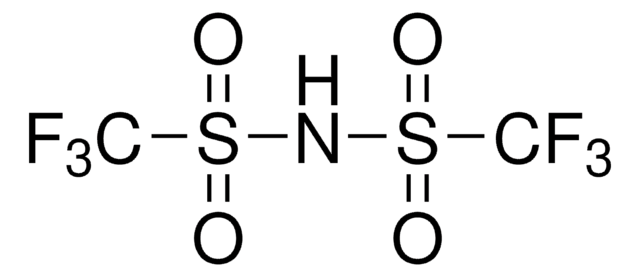
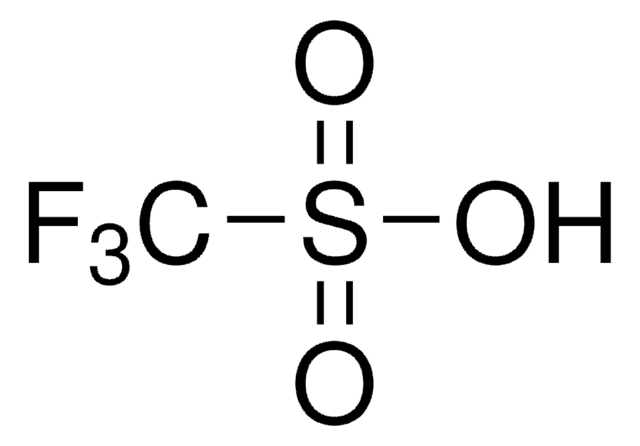
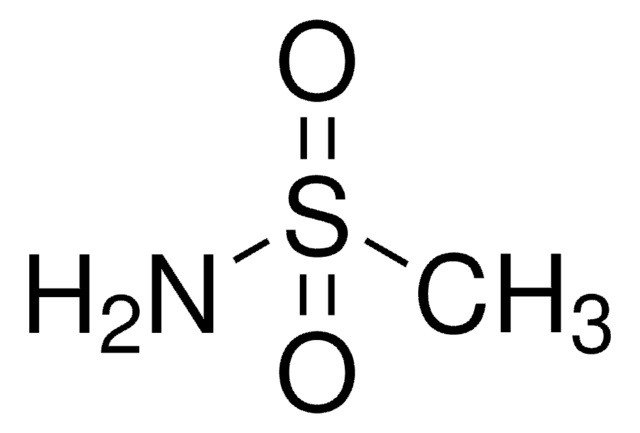
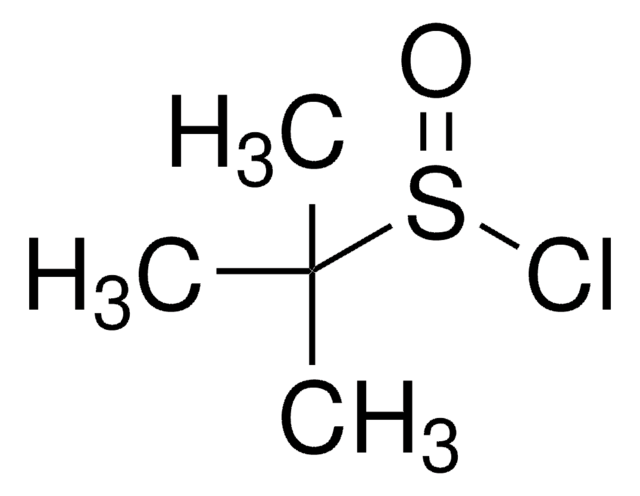
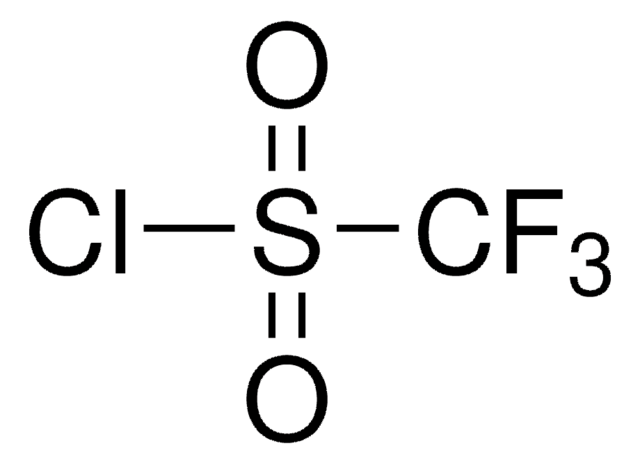
![N-Methyl bis[(trifluoromethyl)sulfonyl]imide ≥90.0% (GC)](/deepweb/assets/sigmaaldrich/product/structures/293/464/eefedfb1-fedb-4509-a339-48035f47c0eb/640/eefedfb1-fedb-4509-a339-48035f47c0eb.png)