57410
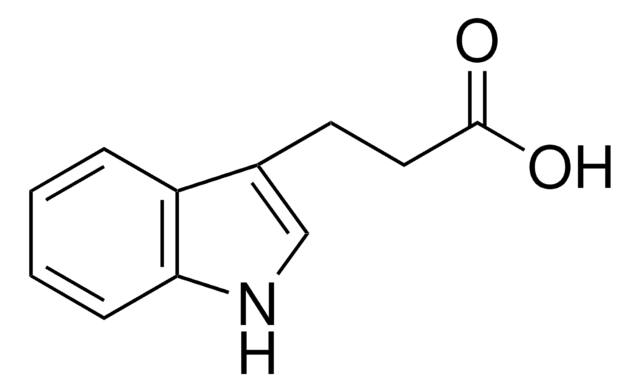
Indole-3-propionic acid
≥97.0% (T)
Synonym(s):
NSC 3252, NSC 47831, 3-(3-Indolyl)propanoic acid, 3-(3-Indolyl)propionic acid, IPA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H11NO2
CAS Number:
Molecular Weight:
189.21
Beilstein:
147733
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (T)
form
solid
SMILES string
OC(=O)CCc1c[nH]c2ccccc12
InChI
1S/C11H11NO2/c13-11(14)6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6H2,(H,13,14)
InChI key
GOLXRNDWAUTYKT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Indole-3-propionic acid can be obtained from tryptophan by deamination reaction.
Application
Reactant for preparation of:
- Fluorescent analogues of strigolactones
- Anti-tumor agents
- Melanocortin receptors ligands
- Immunosuppressive agents
- Iinhibitors of hepatitis C virus
- Histamine H4 receptor agonists
- NR2B/NMDA receptor antagonists
- CB1 antagonist for the treatment of obesity
- Antibacterial agents
- Inhibitor of TGF-β receptor binding
Biochem/physiol Actions
Studied as an adjunct to improve perfusion after liver transplant.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wanguo Wei et al.
Bioorganic & medicinal chemistry letters, 19(24), 6926-6930 (2009-11-10)
New small molecule inhibitors of HCV were discovered by screening a small library of indoline alkaloid-type compounds. An automated assay format was employed which allowed identification of dimerization inhibitors of core, the capsid protein of the virus. These compounds were
Francis Giraud et al.
Bioorganic & medicinal chemistry letters, 20(17), 5203-5206 (2010-07-27)
N-aryl-3-(indol-3-yl)propanamides were synthesized and their immunosuppressive activities were evaluated. This study highlighted the promising potency of 3-[1-(4-chlorobenzyl)-1H-indol-3-yl]-N-(4-nitrophenyl)propanamide 15 which exhibited a significant inhibitory activity on murine splenocytes proliferation assay in vitro and on mice delayed-type hypersensitivity (DTH) assay in vivo.
Ragan, J. A.; et al.
Organic Process Research & Development, 13, 186-186 (2009)
Peng Xu et al.
Bioorganic & medicinal chemistry letters, 17(12), 3330-3334 (2007-04-27)
A series of novel acylide derivatives have been synthesized from erythromycin A via a facile procedure. By applying this procedure, cyclic carbonation to C-11,12 position, acylation to C-3 hydroxyl, and deprotection provided the desired acylides. These compounds showed antibacterial activity
Rosaria Gitto et al.
Bioorganic & medicinal chemistry, 17(4), 1640-1647 (2009-01-23)
A combined ligand-based and structure-based approach has previously allowed us to identify NR2B/NMDA receptor antagonists containing indole scaffold. In order to further explore the main structure activity relationships of this class of derivatives we herein report the design, synthesis and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service