56776
Trihexyltetradecylphosphonium dicyanamide
≥95% (qNMR)
Synonym(s):
Tetradecyltrihexylphosphonium dicyanamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[CH3(CH2)5]3P[N(CN)2](CH2)13CH3
CAS Number:
Molecular Weight:
549.90
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95% (qNMR)
density
0.90 g/mL at 20 °C (lit.)
functional group
amine
phosphine
SMILES string
N#C[N-]C#N.CCCCCCCCCCCCCC[P+](CCCCCC)(CCCCCC)CCCCCC
InChI
1S/C32H68P.C2N3/c1-5-9-13-17-18-19-20-21-22-23-24-28-32-33(29-25-14-10-6-2,30-26-15-11-7-3)31-27-16-12-8-4;3-1-5-2-4/h5-32H2,1-4H3;/q+1;-1
InChI key
DOMOOBQQQGXLGU-UHFFFAOYSA-N
Application
Trihexyltetradecylphosphonium dicyanamide is an ionic liquid that can be used:
- As an extraction solvent for the separation of organic compounds.
- In the experiment to study the solubility of carbon dioxide and binary gas mixtures in ionic liquids.
- To prepare hybrid ionogels having electrochemical applications.
- As a solvent for the extraction of aromatics from pyrolytic sugars.
Caution
may become slightly turbid on storage
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Application of trihexyltetradecylphosphonium dicyanamide ionic liquid for various types of separations problems: Activity coefficients at infinite dilution measurements utilizing GLC method
Kabane B and Redhi GG
Fluid Phase Equilibria, 493, 181-187 (2019)
High pressure solubility of CO2 in non-fluorinated phosphonium-based ionic liquids
Ramdin M, et al.
Journal of Supercritical Fluids, 82, 41-49 (2013)
Solubility of CO2/CH4 gas mixtures in ionic liquids
Ramdin M, et al.
Fluid Phase Equilibria, 375, 134-142 (2014)
Ching-Bin Ke et al.
Nanomaterials (Basel, Switzerland), 8(6) (2018-06-05)
Oxygen and nitrogen capacitively coupled plasma (CCP) was used to irradiate mixtures of aliphatic acids in high boiling point solvents to synthesize fluorescent carbon dots (C-dots). With a high fluorescence intensity, the C-dots obtained from the O₂/CCP radiation of a
Viresh R Thamke et al.
Environmental pollution (Barking, Essex : 1987), 250, 567-577 (2019-04-27)
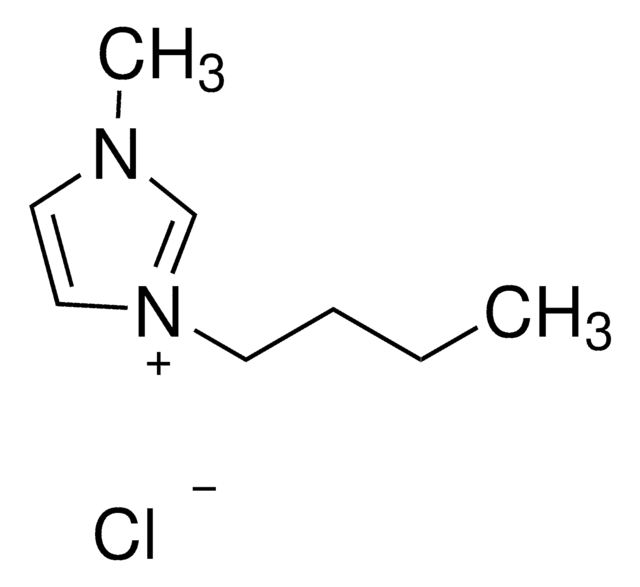
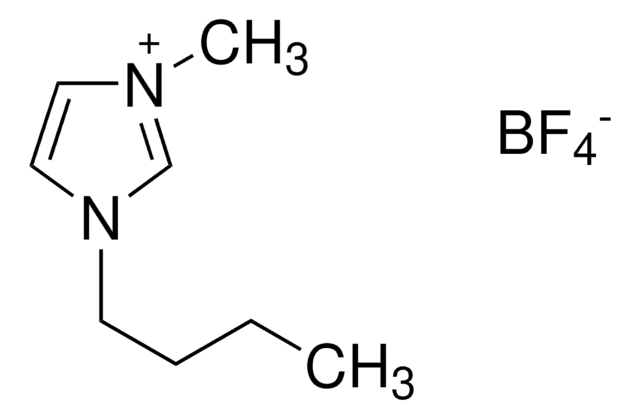
The present study deals with the cyto-genotoxicological impact of ionic liquids, 1-butyl-3-methylimidazolium bromide, trihexyl tetradecylphosphonium dicyanamide, 1-decyl-3-methylimidazolium tetrafluoroborate, benzyldimethyltetradecylammonium chloride, and 1-butyl-4-methylpyridinium chloride, on animal cells and their biodegradation. The long alkyl chain containing ionic liquids were found to be
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service