544639
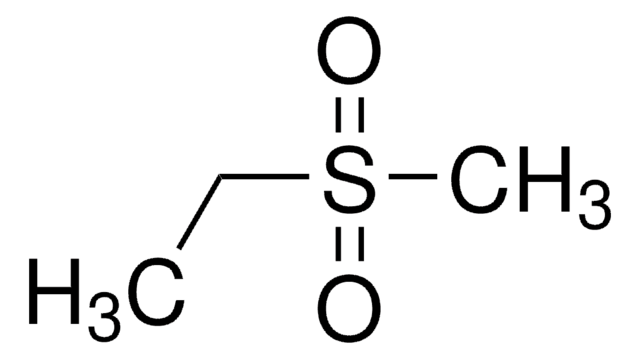
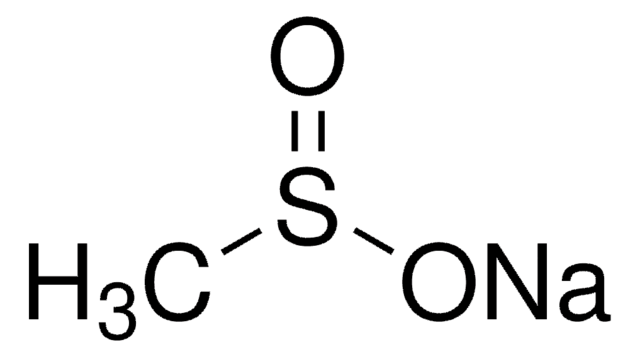
Methanesulfonylacetone
97%
Synonym(s):
1-(Methylsulfonyl)-2-propanone, NSC 35395
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C(O)CH2SO2CH3
CAS Number:
Molecular Weight:
136.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder or crystals
mp
48-52 °C (lit.)
functional group
ketone
sulfone
SMILES string
CC(=O)CS(C)(=O)=O
InChI
1S/C4H8O3S/c1-4(5)3-8(2,6)7/h3H2,1-2H3
InChI key
NWEYGXQKFVGUFR-UHFFFAOYSA-N
General description
Methanesulfonylacetone is a sulfonyl group-containing active methylene compound. It can react with Baylis–Hillman acetates in dimethylformamide/potassium carbonate system to form ortho-hydroxyacetophenone derivatives.
Application
Methanesulfonylacetone may be used in the preparation of 6-bromo-3-methanesulfonyl-2-methyl-quinolin-4-ol.
Reactant for:
- Stereoselective preparation of chiral cyclic ketones
- Preparation of poly-substituted pyridines
- Multicomponent cyclocondensation with aldehydes and aminoazoles
- Gold-catalyzed Friedlander cyclocondensation reaction
- Radical homoallylation reactions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of ortho-hydroxyacetophenone derivatives from Baylis?Hillman acetates.
Kim JN, et al.
Tetrahedron Letters, 43(37), 6597-6600 (2007)
New vistas in quinoline synthesis.
Atechian S, et al.
Tetrahedron, 63(13), 2811-2823 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service