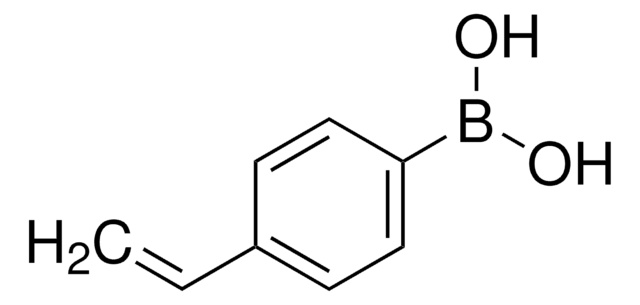
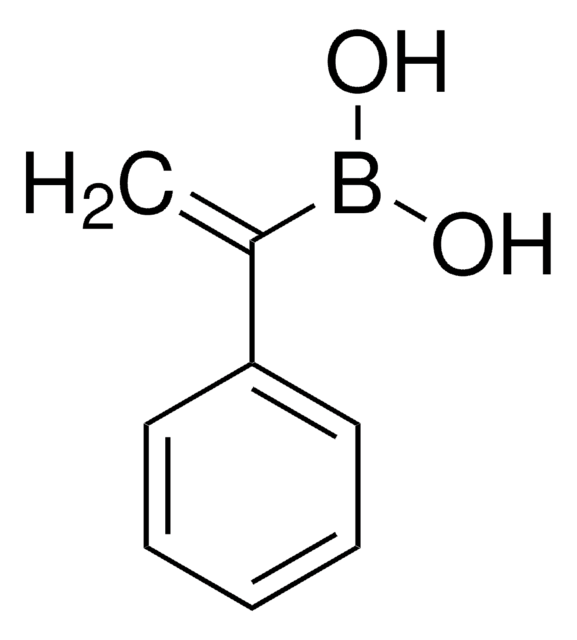
473790
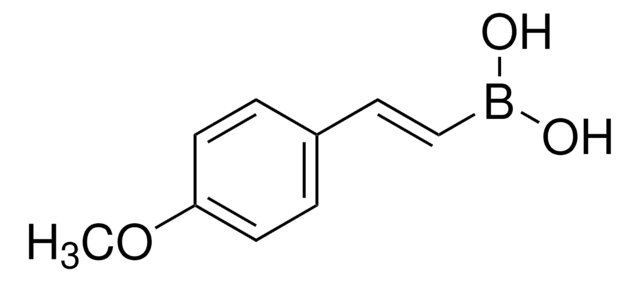
trans-2-Phenylvinylboronic acid
97%
Synonym(s):
(E)-2-phenyl-Etheneboronic acid, (E)-Phenylethenylboronic acid, (E)-Styreneboronic acid, (E)-Styrylboronic acid, trans-(2-Phenylethenyl)boronic acid, trans-Phenylvinyl boronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=CHB(OH)2
CAS Number:
Molecular Weight:
147.97
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
146-156 °C (lit.)
functional group
phenyl
SMILES string
OB(O)\C=C\c1ccccc1
InChI
1S/C8H9BO2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7,10-11H/b7-6+
InChI key
VKIJXFIYBAYHOE-VOTSOKGWSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Reagent used for
Reagent used in Preparation of
- Palladium (Pd)-catalyzed Suzuki-Miyaura coupling reactions
- Rhodium (Rh)-catalyzed intramolecular amination of aryl azides
- Diastereoselective synthesis via Pd-catalyzed Heck-Suzuki cascade reaction
- Copper (Cu)-mediated cyanation
- Rhodium (Rh)-catalyzed asymmetric addition
- Diastereoselective synthesis via iridium (Ir)-catalyzed addition
- Palladium (Pd)-catalyzed cascade cyclization
Reagent used in Preparation of
- Optically active unsaturated amino acids by diastereoselective Petasis borono-Mannich reaction
- Amino alcohol dienes via Petasis 3-component reaction using Ru-catalyzed ring-closing metathesis and isomerization
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tomohiro Iwai et al.
Journal of the American Chemical Society, 134(2), 1268-1274 (2011-12-14)
Iridium complexes show high catalytic activity in intermolecular additions of acid chlorides to terminal alkynes to afford valuable (Z)-β-chloro-α,β-unsaturated ketones. Ligands in the catalytic system play a crucial role in this reaction. An N-heterocyclic carbene (NHC) is an efficient ligand
Xiangqing Feng et al.
Organic letters, 14(2), 624-627 (2012-01-12)
This paper describes a Rh(I)-catalyzed highly efficient and enantioselective 1,2-addition of arylboronic acids to α-diketones with the use of a simple sulfur-alkene hybrid ligand. With as low as a 0.1 mol % catalyst loading, a variety of optically active α-hydroxyketones
Ligand Effects on the Stereochemical Outcome of Suzuki-Miyaura Couplings
Lu, G-P.; et al.
The Journal of Organic Chemistry, 77, 370-3703 (2012)
Erhad Ascic et al.
ACS combinatorial science, 14(4), 253-257 (2012-02-24)
A "build/couple/pair" pathway for the systematic synthesis of structurally diverse small molecules is presented. The Petasis 3-component reaction was used to synthesize anti-amino alcohols displaying pairwise reactive combinations of alkene moieties. Upon treatment with a ruthenium alkylidene-catalyst, these dienes selectively
Diastereoselective synthesis of tetrahydroquinolines via a palladium-catalyzed Heck-Suzuki cascade reaction
Wilson, J. E.
Tetrahedron Letters, 53, 2308-2311 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)