All Photos(1)
About This Item
Empirical Formula (Hill Notation):
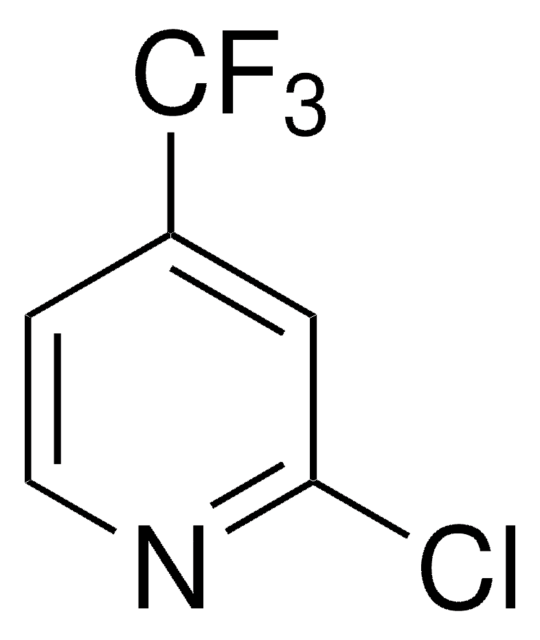
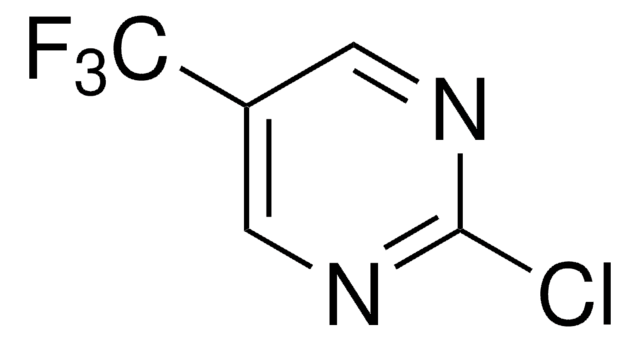
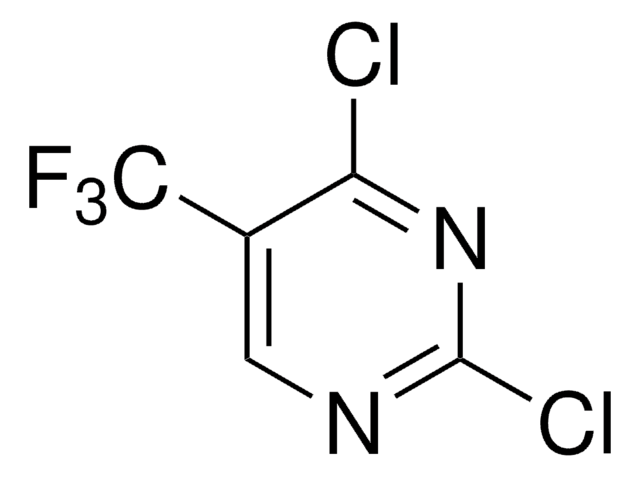
C5H2ClF3N2
CAS Number:
Molecular Weight:
182.53
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.447 (lit.)
bp
60 °C/10 mmHg (lit.)
density
1.513 g/mL at 25 °C (lit.)
functional group
chloro
fluoro
SMILES string
FC(F)(F)c1ccnc(Cl)n1
InChI
1S/C5H2ClF3N2/c6-4-10-2-1-3(11-4)5(7,8)9/h1-2H
InChI key
FZRBTBCCMVNZBD-UHFFFAOYSA-N
General description
2-Chloro-4-(trifluoromethyl)pyrimidine is a pyrimidine derivative. Its density and refractive index have been determined.
Application
2-Chloro-4-(trifluoromethyl)pyrimidine may be used to investigate the effect of chemical substitutions on the interfacial interactions of pyrimidines with the phospholipid-mimic immobilized-artificial-membrane (IAM) chromatographic stationary phase. Monocyclic pyrimidine nucleic acid bases (nucleobases) were reported to behave differently from their bicyclic purine analogs.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
120.2 °F - closed cup
Flash Point(C)
49 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hai-Bin Luo et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 853(1-2), 114-122 (2007-04-03)
The influence of the chemical substitutions on the interfacial interactions of pyrimidines with the phospholipid-mimic immobilized-artificial-membrane (IAM) chromatographic stationary phase was evaluated. Monocyclic pyrimidine nucleic acid bases (nucleobases) were revealed behaving differently from their bicyclic purine counterparts substantially. The computed
Yaws CL.
The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals, 63-63 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service