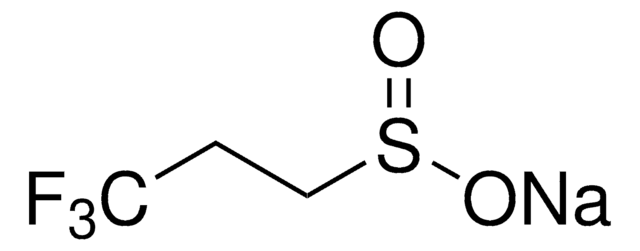
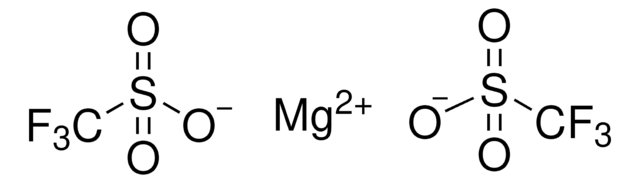367907
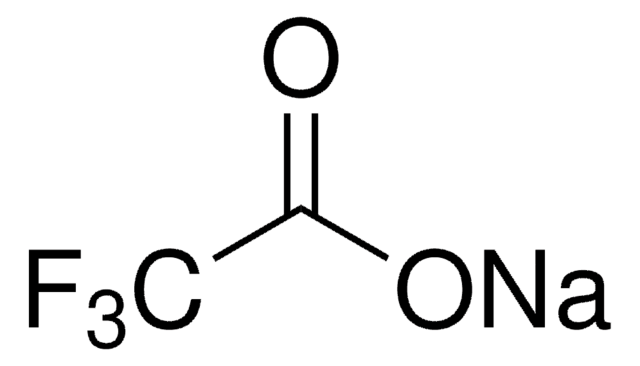
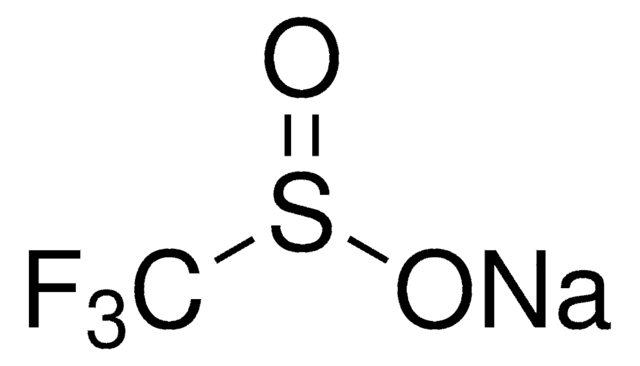
Sodium trifluoromethanesulfonate
98%
Synonym(s):
Sodium triflate, Trifluoromethanesulfonic acid sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CF3SO3Na
CAS Number:
Molecular Weight:
172.06
Beilstein:
3728797
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Assay:
98%
form:
solid
Recommended Products
Quality Level
Assay
98%
form
solid
mp
253-255 °C (lit.)
functional group
fluoro
triflate
SMILES string
[Na+].[O-]S(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S.Na/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
InChI key
XGPOMXSYOKFBHS-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Sodium trifluoromethanesulfonate (Sodium triflate or NaOTf) is an efficient catalyst as well as a reagent in many organic reactions. The prominent application includes catalytic asymmetric Mannich-type reactions, Mannich-type reactions in water, and Diels-Alder reactions.It is also used as an electrolyte in batteries.
Application
Sodium trifluoromethanesulfonate can be employed as a reagent for the preparation of:
It can be also used as supporting electrolyte in electrochemical O-glycosylation of primary alcohols with O-protected thioglycosides.
- Aryl fluorides via silver-catalyzed fluorination of arylstannanes.
- Ionic liquids such as N, N -dialkylpyrrolidinium triflate, N,N-dialkylimidazolium triflate, and N-alkylpyridinium triflate.
It can be also used as supporting electrolyte in electrochemical O-glycosylation of primary alcohols with O-protected thioglycosides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Silver-catalyzed late-stage fluorination
Tang P, et al.
Journal of the American Chemical Society, 132(34), 12150-12154 (2010)
Sodium 1, 1, 1-Trifluoromethanesulfonate
Surya PGK and Mathew T
Encyclopedia of Reagents for Organic Synthesis, Second Edition, 132(34), 12150-12154 (2001)
Chiwon Kang et al.
Materials (Basel, Switzerland), 12(8) (2019-04-26)
High theoretical capacity and low-cost copper sulfide (CuxS)-based anodes have gained great attention for advanced sodium-ion batteries (SIBs). However, their practical application may be hindered due to their unstable cycling performance and problems with the dissolution of sodium sulfides (NaxS)
Bing Sun et al.
Advanced materials (Deerfield Beach, Fla.), 29(48) (2017-04-05)
As a new family member of room-temperature aprotic metal-O
Electrochemical O-glycosylation using thioglycosides as glycosyl donors in the presence of a catalytic amount of sodium trifluoromethanesulfonate as a supporting electrolyte
Tanaka N, et al.
Tetrahedron Letters, 48(41), 7383-7387 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)