364010
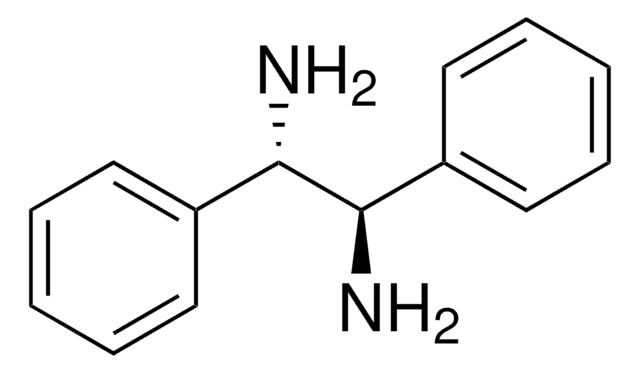
(1R,2R)-(+)-1,2-Diphenylethylenediamine
97%
Synonym(s):
(1R,2R)- DPEDA, (1R,2R)-DPEN, (1R,2R)-(+)-1,2-Diamino-1,2-diphenylethane
About This Item
Recommended Products
Quality Level
Assay
97%
optical activity
[α]20/D +102°, c = 1 in ethanol
optical purity
ee: 99% (GLC)
mp
79-83 °C (lit.)
functional group
amine
phenyl
SMILES string
N[C@@H]([C@H](N)c1ccccc1)c2ccccc2
InChI
1S/C14H16N2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-14H,15-16H2/t13-,14-/m1/s1
InChI key
PONXTPCRRASWKW-ZIAGYGMSSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- (1R,2R)-(+)-1,2-Diphenylethylenediamine (DPEN) is one of the widely used chiral auxiliary and ligand in the synthesis of asymmetric catalysts such as BINAP/diamine-Ru complexes for the stereoselective hydrogenation of ketones.
- It can be used in the preparation of monosulfonyl DPEN-salt to catalyze Michael addition of various ketones to maleimides.
- DPEN-derived chiral triazolium salts can be used to catalyze enantioselective intramolecular Stetter reaction and oxodiene Diels-Alder reaction.
- Zinc acetate complexes of DPEDA-derived ligands can also be used to catalyze hydrosilylation of imines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 364010-1G | 4061831825237 |
| 364010-100MG | |
| 364010-500MG | 4061833038017 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service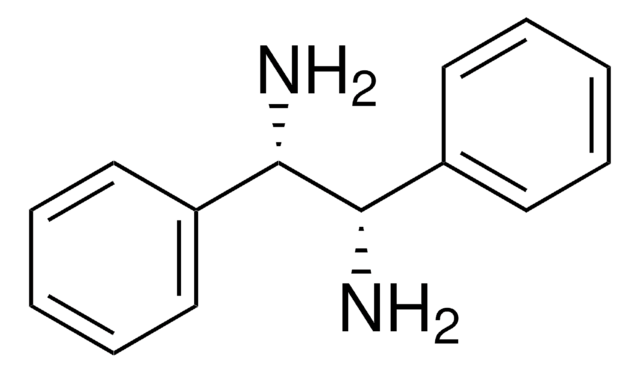



![(1R,2R)-(+)-N,N′-Dimethyl-1,2-bis[3-(trifluoromethyl)phenyl]ethanediamine 97%](/deepweb/assets/sigmaaldrich/product/structures/408/938/05de1ba4-8e30-49a7-996e-99aa9340a1f4/640/05de1ba4-8e30-49a7-996e-99aa9340a1f4.png)