All Photos(1)
About This Item
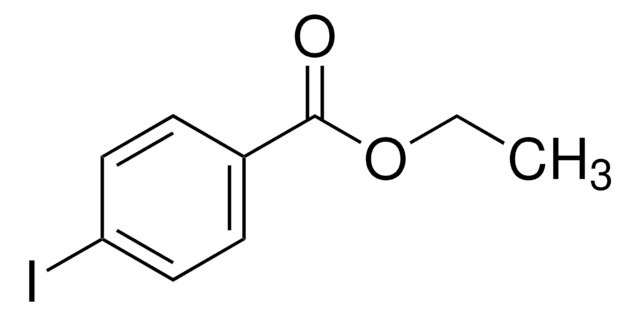
Linear Formula:
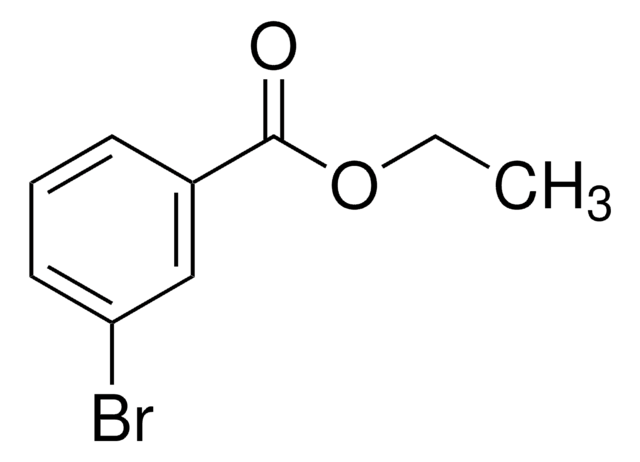
BrC6H4CO2C2H5
CAS Number:
Molecular Weight:
229.07
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.544 (lit.)
bp
131 °C/14 mmHg (lit.)
density
1.403 g/mL at 25 °C (lit.)
functional group
bromo
ester
SMILES string
CCOC(=O)c1ccc(Br)cc1
InChI
1S/C9H9BrO2/c1-2-12-9(11)7-3-5-8(10)6-4-7/h3-6H,2H2,1H3
InChI key
XZIAFENWXIQIKR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl 4-bromobenzoate is an ester having electron-withdrawing substituent. It undergoes reduction with potassium diisobutyl-t-butoxyaluminum hydride (PDBBA) at 0°C to yield aldehydes. Reaction of ethyl 4-bromobenzoate and substituted benzyl chloride with zinc dust and a Pd catalyst is reported.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>235.4 °F - closed cup
Flash Point(C)
> 113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chemoselective Reduction of Esters to Aldehydes by Potassium Diisobutyl-t-butoxyaluminum Hydride (PDBBA).
Chae MJ, et al.
Bull. Korean Chem. Soc., 28(12), 2517-2517 (2007)
Christophe Duplais et al.
Chemical communications (Cambridge, England), 46(4), 562-564 (2010-01-12)
A remarkably simple entry to unsymmetrical diarylmethanes has been developed that relies on an in situ organozinc-mediated, palladium-catalyzed cross-coupling. Thus, by mixing a benzyl and aryl halide together in the presence of Zn metal and a Pd catalyst, diarylmethanes are
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 363774-100G | 4061836684709 |
| 363774-25G | 4061831825190 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service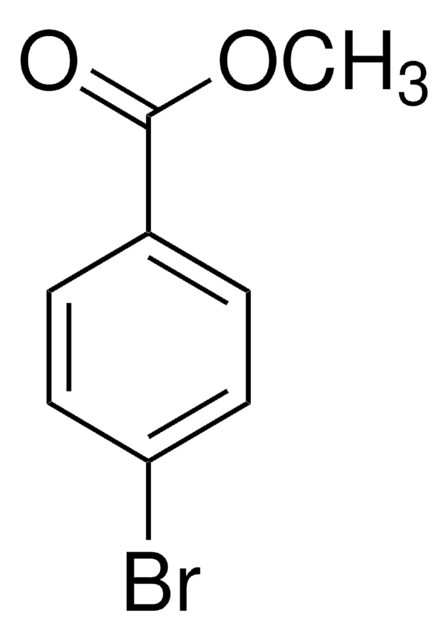



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)