345326
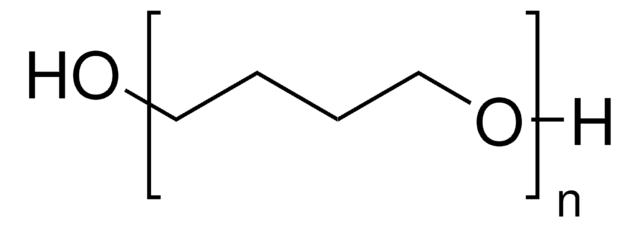
Poly(tetrahydrofuran)
average Mn ~2,000, contains BHT as stabilizer
Synonym(s):
Terathane® 2000 polyether glycol, α-Hydro-ω-hydroxypoly(oxy-1,4-butanediyl), Poly(1,4-butanediol), polyTHF
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H(OCH2CH2CH2CH2)nOH
CAS Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor pressure
<0.01 mmHg ( 25 °C)
<1 mmHg ( 20 °C)
Quality Level
form
solid
mol wt
average Mn ~2,000
contains
BHT as stabilizer
mp
28-40 °C
density
0.972 g/mL at 25 °C
SMILES string
OCCCCO
InChI
1S/C8H18O2/c1-3-5-6-10-8(4-2)7-9/h8-9H,3-7H2,1-2H3/t8-/m0/s1
InChI key
BJZYYSAMLOBSDY-QMMMGPOBSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Poly(tetrahydrofuran) (PTHF) can be used as a macroinitiator to prepare block copolymers via ring-opening polymerization.
Cross-linked PTHF can be used as a matrix to prepare thermally stable polymer electrolytes for lithium-ion batteries.
It can also be used as a starting material to prepare cyclodextrin-basedsupramolecular complexes for drug and gene delivery.
Cross-linked PTHF can be used as a matrix to prepare thermally stable polymer electrolytes for lithium-ion batteries.
It can also be used as a starting material to prepare cyclodextrin-basedsupramolecular complexes for drug and gene delivery.
Legal Information
Product of DuPont
Terathane is a registered trademark of Invista North America S.a.r.l.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Renier et al.
Journal of biomaterials science. Polymer edition, 5(3), 231-244 (1993-01-01)
Poly(etherurethane urea) (PEUU) elastomers when employed as biomedical devices may be susceptible to extraction upon implantation. Four PEUU elastomers containing a single PEUU formulation, but varying in terms of their additives, were subjected to an in vitro extraction procedure. The
P Banu et al.
Journal of colloid and interface science, 277(2), 304-308 (2004-09-03)
Aqueous dispersions of poly(ester-imide)s [P(E-I)s] have been prepared by dispersing the P(E-I)s in water without any external solubilizing agents. P(E-I)s were prepared from anhydride-terminated polyester prepolymer and diisocyanate. The -COOH groups in the polymer were then neutralized using triethylamine and
Antony Memboeuf et al.
Journal of the American Society for Mass Spectrometry, 22(10), 1744-1752 (2011-09-29)
Collision induced dissociation tandem mass spectrometry experiments were performed to unequivocally separate compounds from an isobaric mixture of two products. The Survival Yield curve was obtained and is shown to consist in a linear combination of the curves corresponding to
W K Loke et al.
Biomaterials, 17(22), 2163-2172 (1996-11-01)
Hybrid biomaterials have been produced by the interaction of polyurethane oligomers with both fresh and glutaraldehyde-fixed porcine pericardium. The hybrid biomaterials so formed were translucent with occasional white streaks and/or spots, had increased stiffness (to touch) but remained pliable. No
R S Labow et al.
Journal of biomaterials science. Polymer edition, 8(10), 779-795 (1997-01-01)
Although biodegradation of model poly(ester-urethane)s and poly(ether-urethane)s has been demonstrated using a single enzyme system (cholesterol esterase (CE) in vitro, in vivo biodegradation most likely involves many processes acting together. In this study, the physical (film vs textured surface) and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service