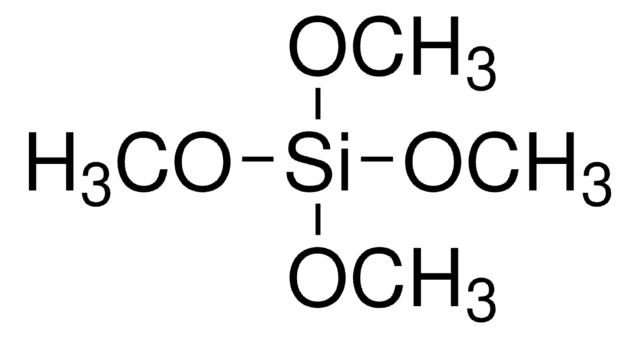341436
Tetramethyl orthosilicate
≥99%
Synonym(s):
Tetramethoxysilane
About This Item
Recommended Products
vapor density
5.25 (vs air)
Quality Level
vapor pressure
13 hPa ( 20 °C)
Assay
≥99%
form
liquid
refractive index
n20/D 1.368 (lit.)
bp
121-122 °C (lit.)
mp
−4 °C (lit.)
density
1.023 g/mL at 25 °C (lit.)
SMILES string
CO[Si](OC)(OC)OC
InChI
1S/C4H12O4Si/c1-5-9(6-2,7-3)8-4/h1-4H3
InChI key
LFQCEHFDDXELDD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a precursor to synthesize organic-inorganic coating materials by sol-gel processing method.
- As an efficient reagent for direct amidation of aliphatic and aromatic carboxylic acids with amines and anilines.
- As a precursor to fabricate SiO2 nanocomposite films by chemical vapor deposition(CVD) method.
- As a selective catalyst ofC3-methylation of indole.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
78.8 °F - closed cup
Flash Point(C)
26 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Research involving reactive silicone chemistry has focused on the production of pure silicon and hybrid materials, hydrosilylation, ring-opening and atom transfer polymerizations, polymerizations with controlled stereochemistry, and condensation reactions.
Silica is a very popular inorganic nanomaterial used in a wide range of applications including fillers for rubber, catalyst supports, separation media, carriers in food and agriculture, and abrasive/anticaking agents in cosmetics. It is also widely believed to be an important material for biomedical applications for following reasons.
Synthesis of Melting Gels Using Mono-Substituted and Di-Substituted Alkoxysiloxanes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service