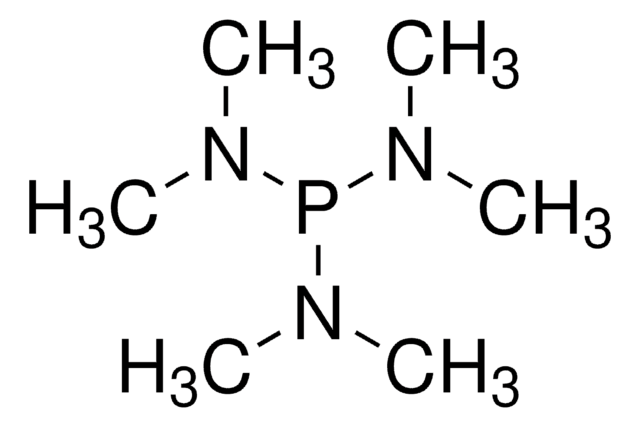
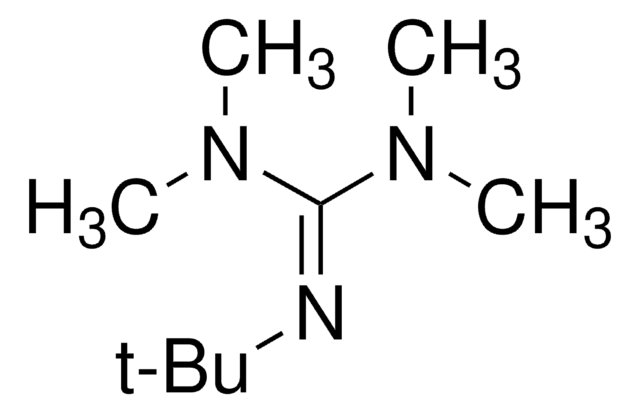
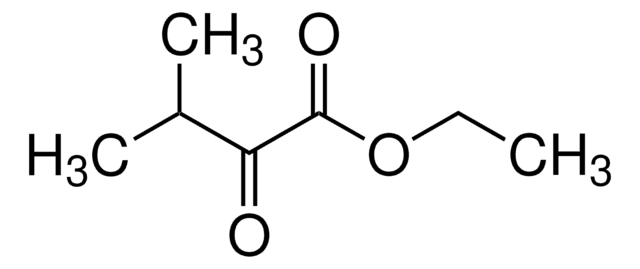
258911
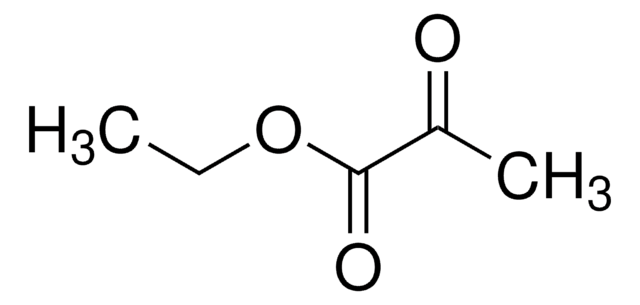
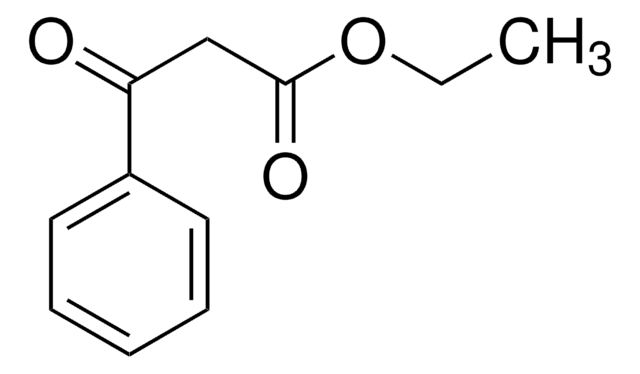
Ethyl benzoylformate
95%
Synonym(s):
Ethyl phenylglyoxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
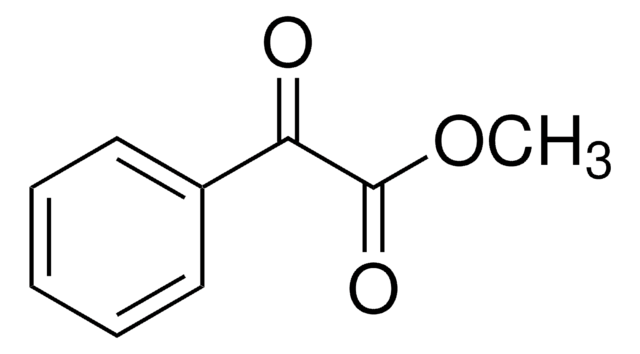
C6H5COCO2C2H5
CAS Number:
Molecular Weight:
178.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.516 (lit.)
bp
138-139 °C/18 mmHg (lit.)
density
1.122 g/mL at 25 °C (lit.)
functional group
ester
ketone
phenyl
SMILES string
CCOC(=O)C(=O)c1ccccc1
InChI
1S/C10H10O3/c1-2-13-10(12)9(11)8-6-4-3-5-7-8/h3-7H,2H2,1H3
InChI key
QKLCQKPAECHXCQ-UHFFFAOYSA-N
Related Categories
General description
Enantioselective hydrogenation of ethyl benzoylformate on Pt/Al2O3 modified with dihydrocinchonidine has been studied.
Application
Ethyl benzoylformate has been used in preparation of 1,5-dihydro-5-deazaflavin derivatives possessing a chiral substituent at N(3) position.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of chiral 5-deazaflavin derivatives and their asymmetric reduction of ethyl benzoylformate.
Tanaka K, et al.
Tetrahedron Letters, 25(16), 1741-1742 (1984)
98% Enantioselectivity in the asymmetric synthesis of a useful chiral building block by heterogeneous method: Enantioselective hydrogenation of ethyl-benzoylformate over cinchona modified Pt/Al2 O3 catalysts in the acetic acid.
Sutyinszki M, et al.
Catalysis Communications, 3(3), 125-127 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service